SO3 Lewis Structure (Sulfur Trioxide) YouTube
A step-by-step explanation of how to draw the SO3 Lewis Dot Structure (Sulfur trioxide).For the SO3 structure use the periodic table to find the total number.

Draw The Lewis Dot Structure For So3 2 slidesharedocs
The sulfur trioxide is a tetra atomic chemical molecule where both the sulfur and three oxygen molecules bond with an equal number of valence electrons. The diagram is drawn showing dots of valence electrons around the symbol of both sulfur and oxygen atoms with lines predicting bond formation.
steps of drawing SO3 lewis structure VSEPR method
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".
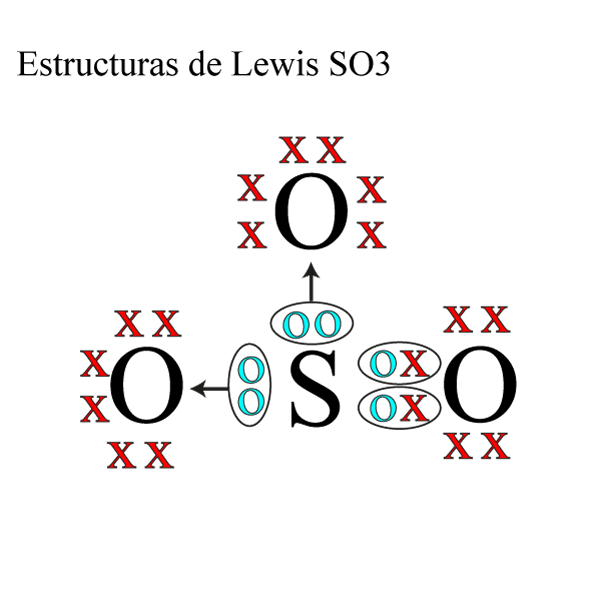
Estructura de Lewis SO3 » Quimica Online
0:00 / 4:53 Lewis Structure of SO3 (Sulfur Trioxide) chemistNATE 259K subscribers Subscribe Subscribed 4.9K Share 625K views 9 years ago Lewis Structures How to draw the Lewis Structure of.
LEWIS DIAGRAM FOR SO3 YouTube
Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to have the correct number of electrons. If the species is an ion, add or subtract electrons corresponding to the charge of the ion. Add an electron for every negative (-) charge, and.

make structure of hybridization of SO3 Brainly.in
SO3 Lewis Structure - Sulfur Trioxide The Organic Chemistry Tutor 7.24M subscribers Subscribe Subscribed 607 70K views 3 years ago This chemistry video explains how to draw the Lewis.

How to draw SO3 Lewis Structure? Science Education and Tutorials
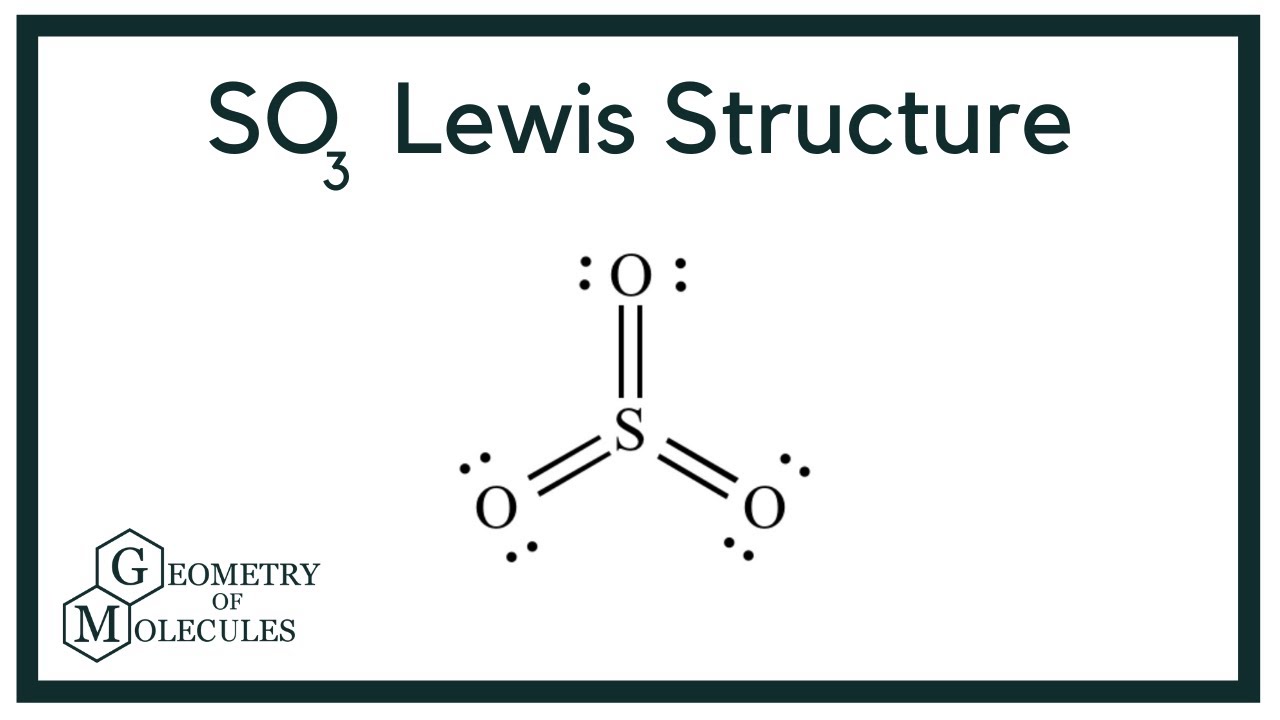
Lewis structure of SO3 (or Sulfur trioxide) contains three double bonds between the Sulfur (S) atom and each Oxygen (O) atom. The Sulfur atom (S) is at the center and it is surrounded by 3 Oxygen atoms (O). The Sulfur atom does not have a lone pair while all the three Oxygen atoms have 2 lone pairs.

Estructura de Lewis SO3 » Quimica Online
The SO3 Lewis structure shows a central Sulfur (S) atom with three Oxygen (O) atoms around it. These atoms are connected by double bonds, and each Oxygen atom has two lone pairs of electrons. In this page, you'll find a detailed, step-by-step guide on how to draw the Lewis structure for SO3. Step-by-Step Guide to […]

Draw the Lewis dot structure for SO3 Brainly.in
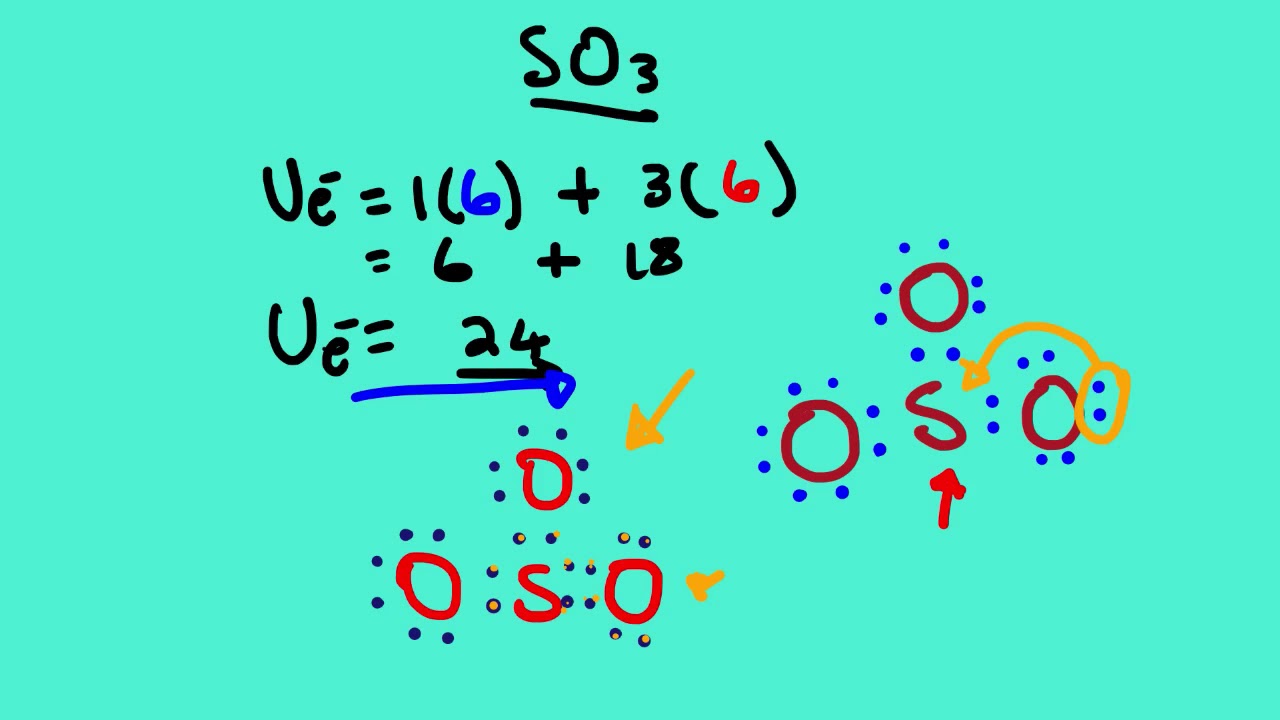
-the physical properties of a molecule such as boiling point, surface tension, etc. Drawing the Lewis Structure for SO 3 ( Sulfur Trioxide) SO 3 is the primary contributer to acid rain in the atomsphere. It is a form of pollution. SO 3 is named Sulfur Trioxide. There are 32 valence electrons available for the Lewis structure for SO 3.

SO3 Lewis Structure, Molecular Geometry, and Hybridization Techiescientist
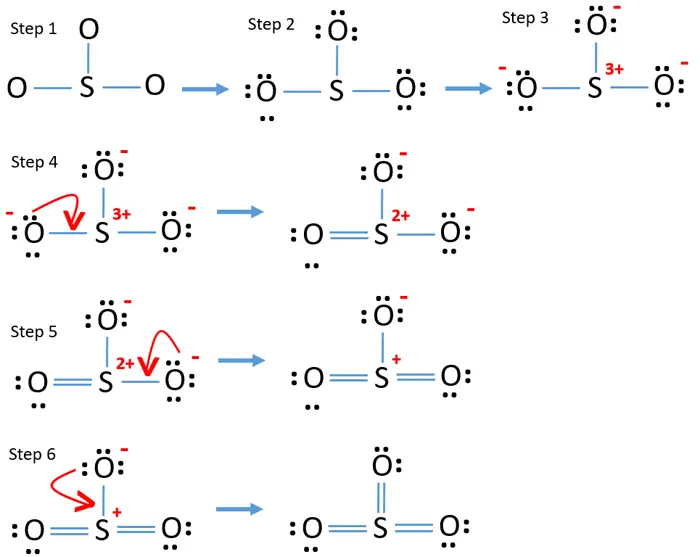
To draw this structure, begin by sketching a rough diagram of the molecular arrangement. Next, indicate the lone pairs on each atom and check for any formal charges. If formal charges are present, convert lone pairs to minimize these charges. Repeat this process until all charges are minimized.

SO3 Lewis StructureLewis structure of SO3 (Sulfur trioxide) YouTube
Steps of drawing SO3 lewis structure Step 1: Find the total valence electrons in SO3 molecule. In order to find the total valence electrons in SO3 (sulfur trioxide) molecule, first of all you should know the valence electrons present in sulfur atom as well as oxygen atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.)

SO3 Lewis Structure, Molecular Geometry, and Hybridization Techiescientist
0:00 / 2:55 SO3 2- Lewis Structure - How to Draw the Lewis Structure for SO3 2- (Sulfite Ion) Wayne Breslyn 724K subscribers Join Subscribe Subscribed 1.9K Share 411K views 10 years ago.

Lewis Dot Structure For So3 slidesharedocs
Sulfur trioxide (alternative spelling sulphur trioxide, also known as nisso sulfan) is the chemical compound with the formula SO 3. It has been described as "unquestionably the most important economically" sulfur oxide. [1] It is prepared on an industrial scale as a precursor to sulfuric acid .

So3 Lewis Structure With Formal Charges
The SO3 Lewis structure shows a central Sulfur (S) atom with three Oxygen (O) atoms around it. These atoms are connected by double bonds, and each Oxygen atom has two lone pairs of electrons. In this page, you'll find a detailed, step-by-step guide on how to draw the Lewis structure for SO3. Step-by-Step Guide to Drawing the Lewis Structure of SO3

SO3 Lewis Structure How to Draw the Lewis Structure for SO3 (Sulfur Trioxide) YouTube
Sulfur trioxide is a oxide of sulfur and colourless inorganic gas. Also it is a toxic gas. Sulfur trioxide gas is produced due to oxidation of sulfur dioxide gas in air. Lewis structure of SO 3 molecule There are three double bonds around sulfur atom with oxygen atoms in SO molecule. Each oxygen atom has two lone pairs in SO 3 lewis structure.
Lewis Dot Diagram For So3 Wiring Diagram
Website-http://www.kentchemistry.com/links/bonding/LewisDotTutorials/SO3.htmI quickly take you through how to draw the Lewis Structure of SO3 (Sulfur Trioxid.