
CO2 Lewis Structure ,Valence Electrons, Formal Charge ,Polar or
Lewis Structure of CO2. CO 2 has a total of 16 valence electrons (four for carbon and two for oxygen), which are organized as O=C=O. In their outermost shells, both oxygen and carbon atoms require 8 electrons to complete an octet. Both oxygen atoms share two electrons with the carbon atom to form two double bonds (O=C), which can also be.

CO2 Lewis Structure ,Valence Electrons, Formal Charge ,Polar or
The Lewis structure of carbon dioxide (CO2) consists of a central carbon atom bonded to two oxygen atoms. The carbon atom shares two electrons with each oxygen atom, forming double bonds. This results in a linear molecular geometry for CO2.

CO2 Lewis Structure ,Valence Electrons, Formal Charge ,Polar or
Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to have the correct number of electrons. If the species is an ion, add or subtract electrons corresponding to the charge of the ion. Add an electron for every negative (-) charge, and.
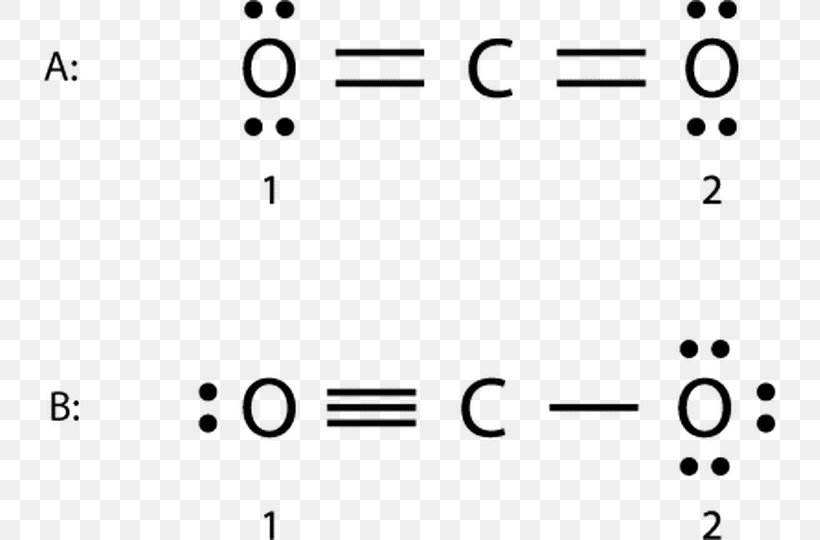
Co2 Dot Structure
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

Draw The Lewis Structure Of Carbon Dioxide Co2 Fotodtp
For Lewis structure of CO2, you will now have two Oxygen atoms forming double bonds with a Carbon atom. As all the valence electrons of all the atoms are used, there are no lone pairs of electrons or non-bonding pairs of electrons in the molecule. To further understand the molecular geometry of CO2, let us quickly go through its hybridization.

CO2 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagram
The N atom has the following Lewis electron dot diagram: It has three unpaired electrons, each of which can make a covalent bond by sharing electrons with an H atom. The electron dot diagram of NH 3 is as follows: Exercise 12.4.2 12.4. 2. Use a Lewis electron dot diagram to show the covalent bonding in PCl 3. Answer.

CO2 Lewis Structure ,Valence Electrons, Formal Charge ,Polar or
A step-by-step explanation of how to draw the CO2 Lewis Dot Structure (Carbon dioxide).For the CO2 structure use the periodic table to find the total number.
Co Molecule Lewis Structure
Lewis Structure Finder. This widget gets the Lewis structure of chemical compounds. Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.

What is the Lewis Dot structure for CO2 (Carbon dioxide)?
The Lewis dot structure of carbon dioxide is shown in Figure \(\PageIndex{8}\). Figure \(\PageIndex{8}\): Three resonance structures of carbon dioxide. In the above Figure we see the second and third resonance structures average out to the first, and so the average of all the resonance structures is a double bond. Thus it is common to write.

Carbon Lewis Dot Structure
The CO 2 Lewis structure depicts the molecular arrangement of carbon dioxide, which is composed of one carbon atom and two oxygen atoms. Within the CO 2 Lewis structure, the carbon atom is surrounded by two double bonds, with each oxygen atom attached to it. Additionally, there are two lone pairs on each oxygen atom. To draw a CO 2 Lewis structure, begin by sketching out a rough structure of.

What is the Lewis Dot structure for CO2 (Carbon dioxide)?
CO2 Lewis Structure. The lewis structure of CO2 can be with some simple steps, but before that, it is important to understand lewis structure properly. So lewis structure generally gives us an idea about the nature of bonding and octet fulfillment of the atoms. According to the octet rule, an atom attains stability by fulfilling its octet.

CO2 Lewis Structure How to Draw the Dot Structure for Carbon Dioxide
Lewis Structure of Carbon Dioxide. Carbon dioxide is a colourless, odourless, incombustible gas produced by the combustion of carbon. The carbon-oxygen ratio in a CO 2 molecule is 1:2. Two double bonds connect the carbon and oxygen atoms in the Lewis structure. Two oxygen atoms are present at the terminals, where they share electrons and form.

Resonance Structures for CO2 (Carbon dioxide) YouTube
Both carbon monoxide, CO, and carbon dioxide, CO 2, are products of the combustion of fossil fuels. Both of these gases also cause problems:. The Lewis structure of XeF 2 shows two bonding pairs and three lone pairs of electrons around the Xe atom: XeF 6: We place three lone pairs of electrons around each F atom, accounting for 36 electrons.

Carbon Dioxide Electron Dot Diagram General Wiring Diagram
A step-by-step explanation of how to draw the Lewis Dot Structure for Carbon dioxide (CO2 ).For the Carbon dioxide structure we use the periodic table to fi.
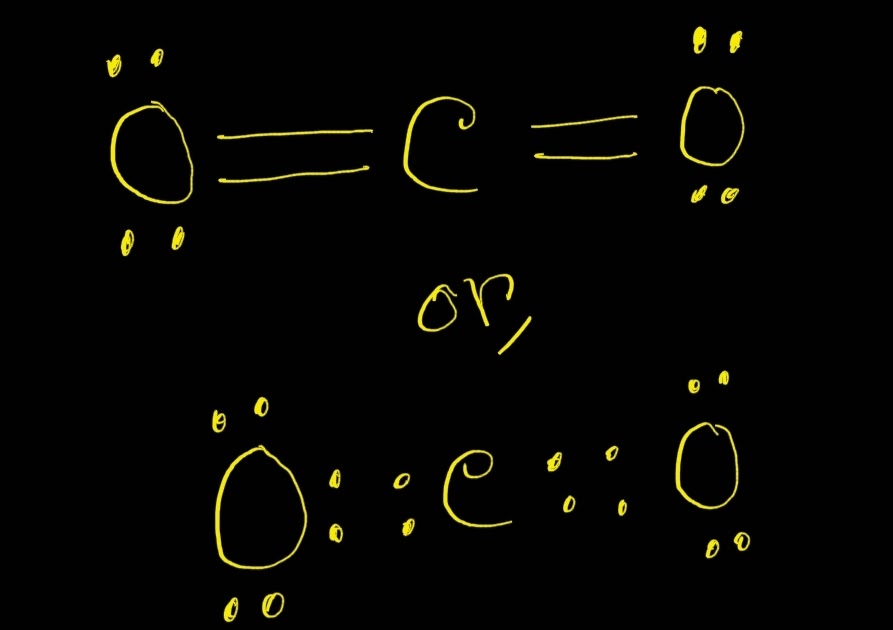
CO2 Lewis Structure ,Valence Electrons, Formal Charge ,Polar or
Carbon dioxide (CO 2) lewis structure has two oxygen atoms and one carbon atom. There are two double bonds around carbon atom in the CO 2. No lone pairs on carbon atom and each oxygen atom has two lone pairs on their valence shells. Shape of CO 2 is linear. Steps of drawing the lewis structure of CO 2 are explained in detail in this tutorial.
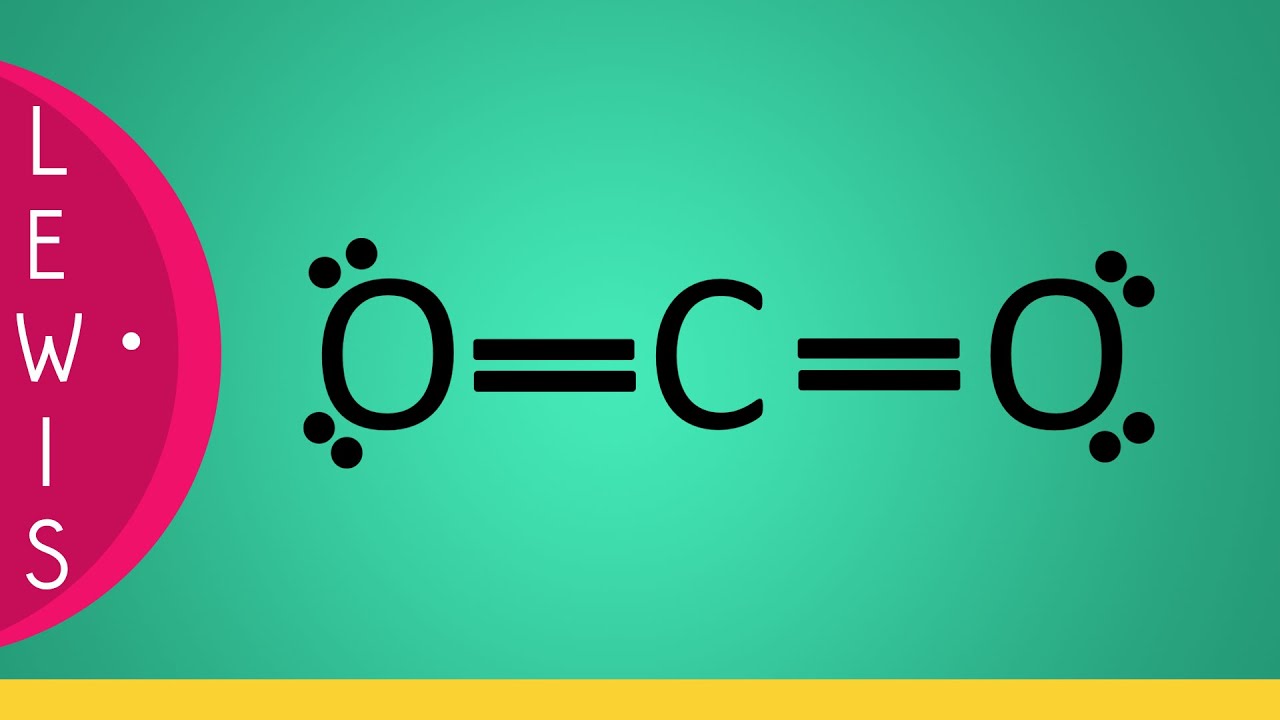
CO2 Lewis Structure YouTube
This chemistry video explains how to draw the lewis structure of CO2 also known as Carbon Dioxide. It also discusses the bond angle, molecular geometry, and.