
C2H5OH Lewis structure, molecular geometry, hybridization, bond angle
The total number of valence electrons available for drawing ethanol (C2H5OH) Lewis structure is 20. C 2 H 5 OH has an identical electron and molecular geometry or shape, i.e., tetrahedral. The C 2 H 5 OH molecule has sp 3 hybridization. The bonded atoms form a mutual bond angle of 109.5° in the tetrahedral C 2 H 5 OH molecule.

C2H5OH Lewis Structure (Ethanol) YouTube
SOLVED: Give the IUPAC name and draw the Lewis and 3D structure of C2H5OH. . Snapsolve any problem by taking a picture. Try it in the Numerade app? VIDEO ANSWER: Right level of structure. There are names for the awesome power of C four H nine. We can grow only three customers of the C four H ninecl if you get this first and the total is dizzy.

C2H5OH (Ethanol) Molecular Geometry and Bond Angles YouTube
Use information from step 4 and 5 to draw the lewis structure. Lewis dot structure of C 2 H 5 OH. Alternatively a dot method can be used to draw the lewis structure. Calculate the total valence electrons in the molecule. C:4x2=8 H:1x6=6 O:6. Total=20 i.e . 10 pairs
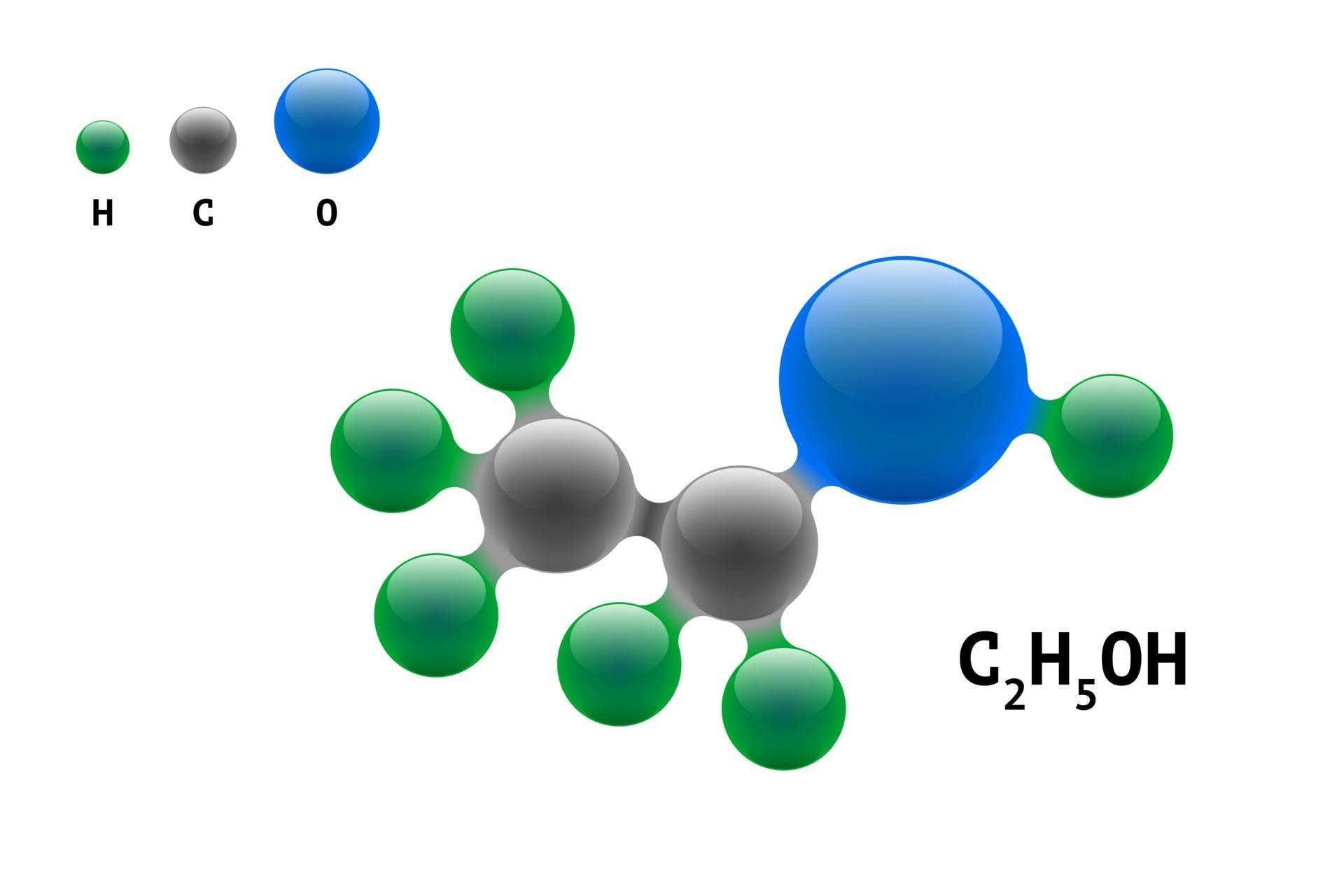
Chemistry model molecule ethanol C2H5OH scientific element formula
Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. For math, science, nutrition, history.
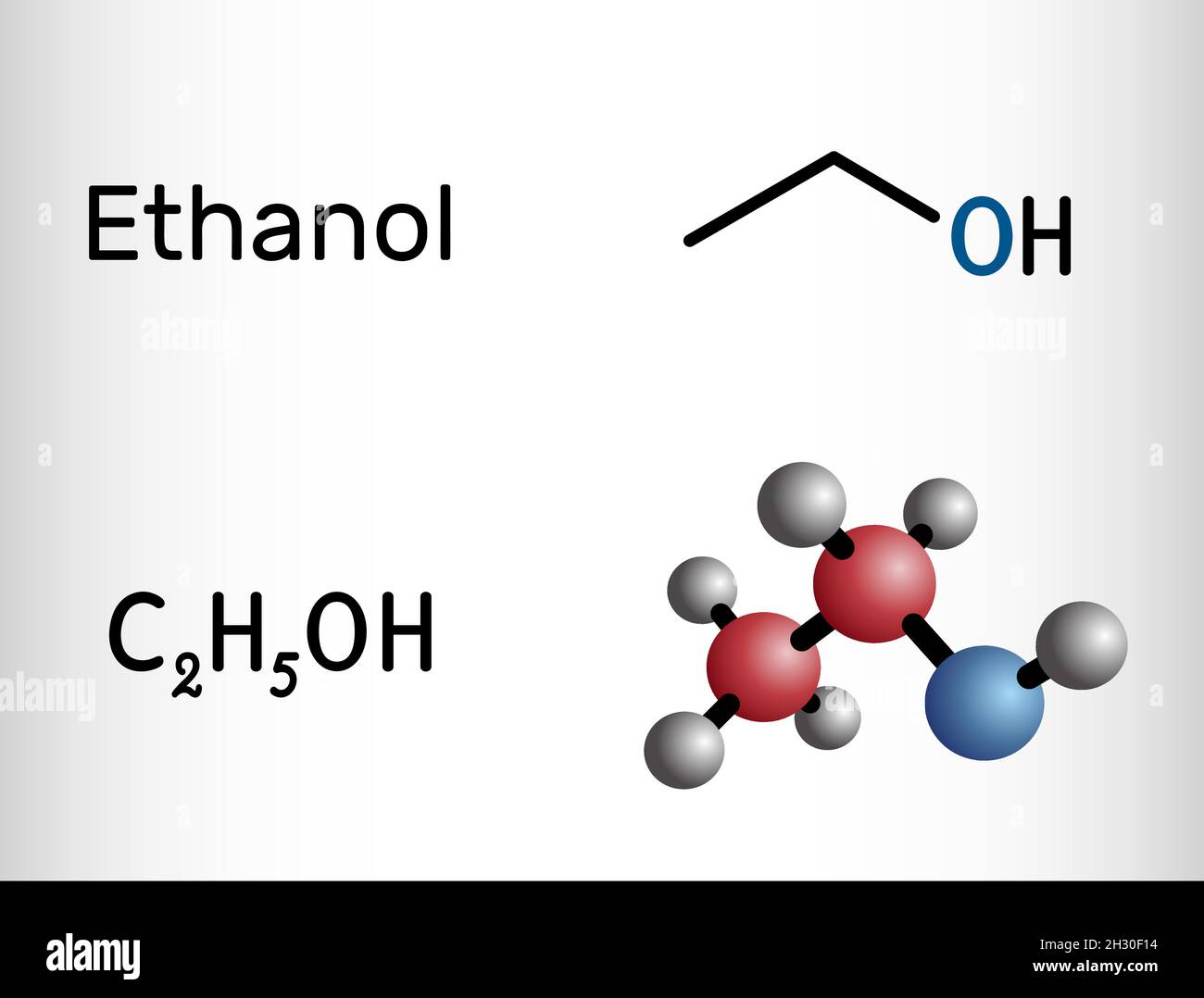
Éthanol, molécule C2H5OH.C'est un alcool primaire, un alcool alkyle
Lewis Structure Finder. This widget gets the Lewis structure of chemical compounds. Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.

Lewis Structure For Ethanol
Steps of drawing C2H5OH lewis structure Step 1: Find the total valence electrons in C2H5OH molecule. In order to find the total valence electrons in a C2H5OH molecule, first of all you should know the valence electrons present in carbon atom, hydrogen atom as well as oxygen atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.)

Draw the electron dot structure of Ethanol ( C2H5OH). Brainly.in
Learn to determine if C2H5OH (Ethanol) is polar or non-polar based on the Lewis Structure and the molecular geometry (shape).We start with the Lewis Structur.

Ethanol 3D Modell C2H5OH 3DModell 5 .3ds .fbx .max .unknown .obj
Ethanol (C2H5OH) is a polar molecule because it has poles of partial positive charge (ẟ+) and partial negative charge (ẟ-) on it. Let me explain this to you in 2 steps! Step #1: Draw the lewis structure. Here is a skeleton of C2H5OH lewis structure and it contains C-H bonds, C-O bond, C-C bond and O-H bond.
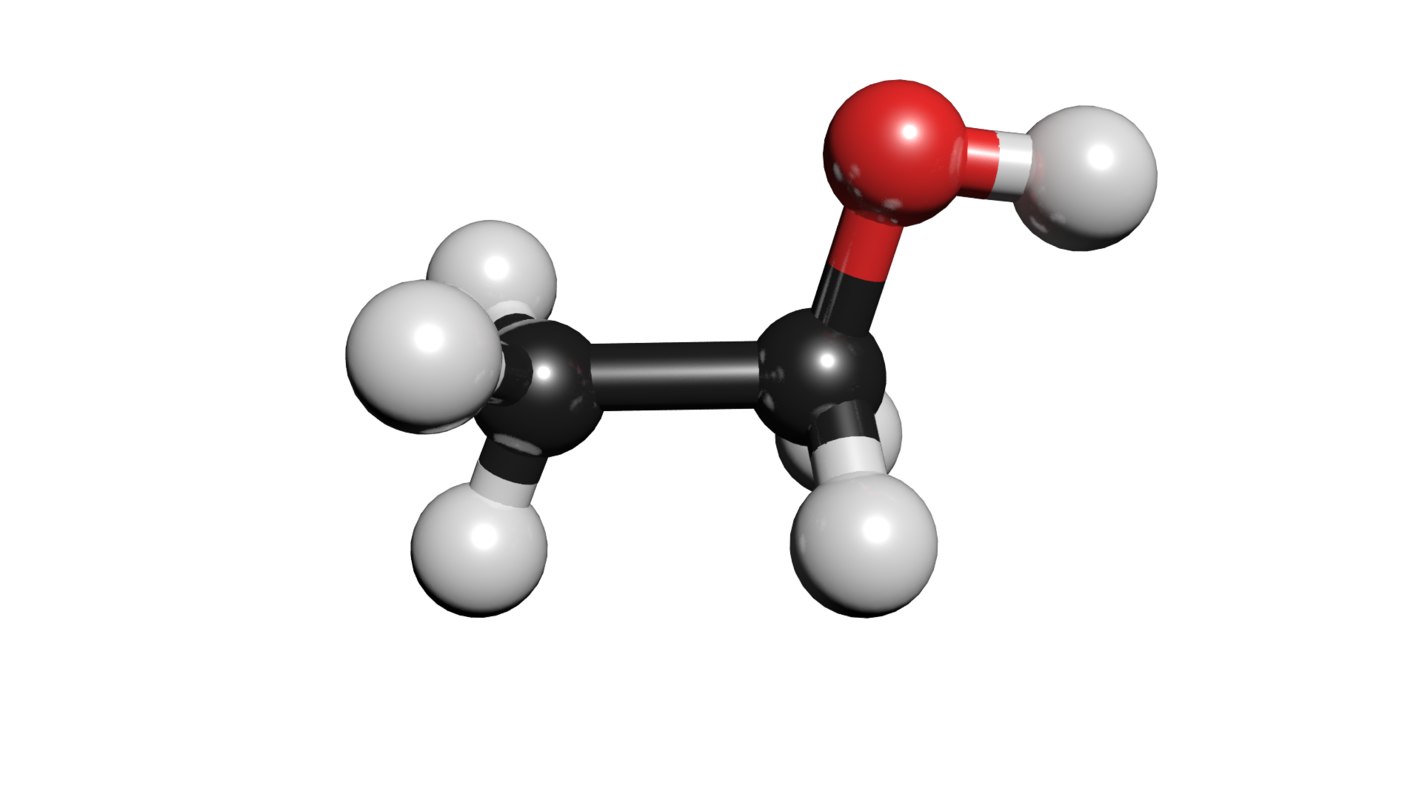
C2h5oh molecule ethanol 3D TurboSquid 1424061
Lewis structure of C2H5OH (or Ethanol) contains five C-H bonds, one O-H bond and one C-O bond. The two Carbon atoms (C) are at the center and it is surrounded by Hydrogen atoms (H) and one OH group. The oxygen atom has 2 lone pairs. Let's draw and understand this lewis dot structure step by step. (Note: Take a pen and paper with you and try.

Ethanol 3D Modell C2H5OH 3DModell 5 .3ds .fbx .max .unknown .obj
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".
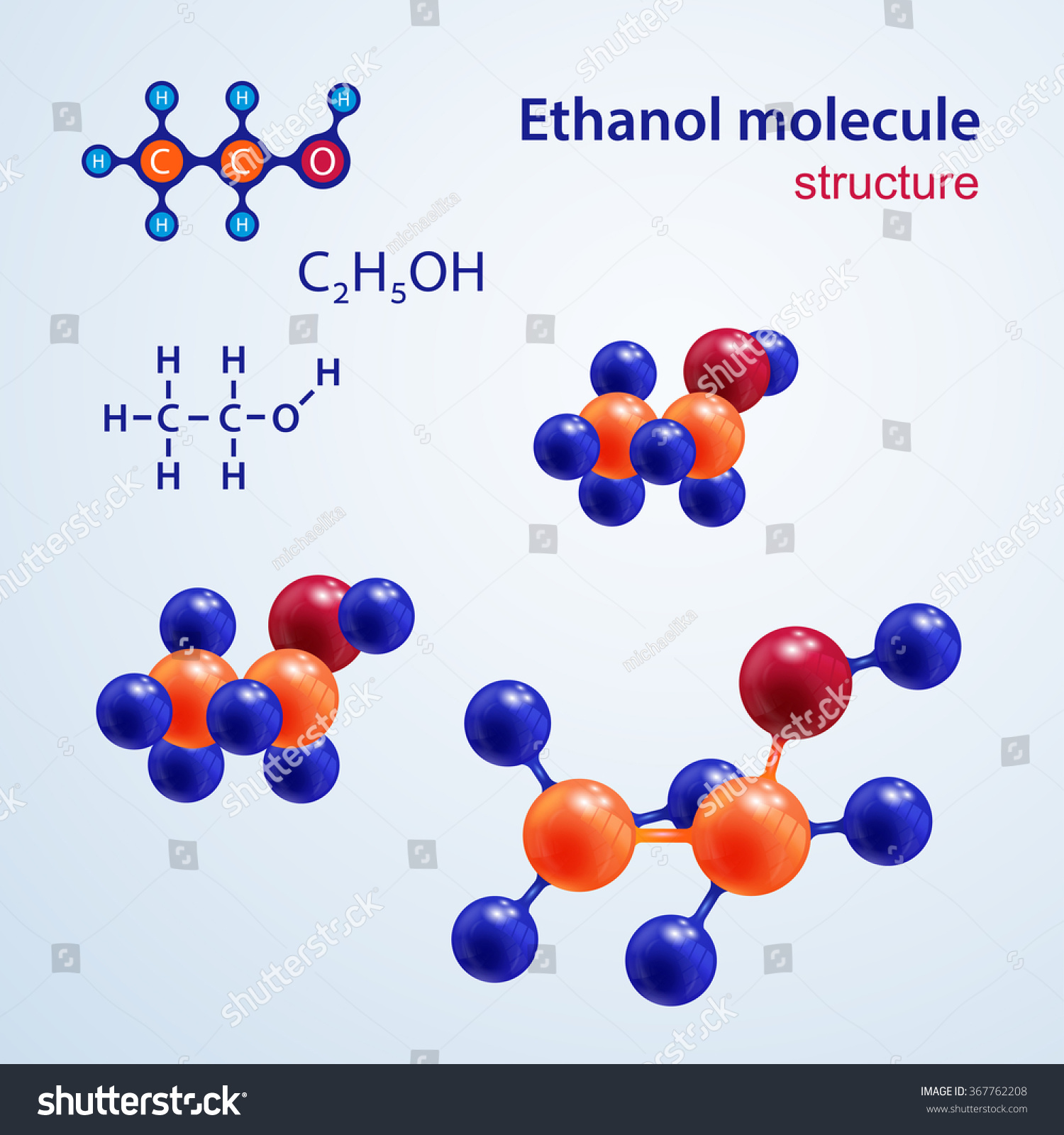
Structure Molecules Ethanol Molecule Chemical Structural Stock Vector
Lewis Structure Molecular Formula Condensed Formula Functional Group (s) Compound Type (alkane, alcohol, etc.) 3D Representation (Use lines, wedges, and dashes.) Geometry Around the Carbon Atoms (linear, trigonal planar, or tetrahedral) H CH H | Alkens HC H H2CH2 H Joaca ------ Alkane 354Spahubirea Trigonal planak 46-5p3 Hybridisch Teteanedral.

Semless Pattern with C2H5OH Medical Chemical Formula of Alcohol Ethanol
Ethanol is a chemical compound that is commonly used as a solvent, fuel, and in the production of alcoholic beverages. Its molecular formula is C2H5OH, and it consists of two carbon atoms, six hydrogen atoms, and one oxygen atom. The Lewis dot structure of ethanol shows the arrangement of these atoms and their valence electrons.

C2h5oh Lewis Dot Structure
Physical properties of Ethanol: The boiling point of Ethanol ( C2H5OH) is 78.5 °C. The melting point of Ethanol is -114.5 °C. The molecular weight of Ethanol is 46.06 g/mol. It is an excellent solvent and is also known as grain alcohol or alcohol. It has a pleasant odour.

draw the structure of ethanol molecule howtotieashirtknotforkids
An explanation of the molecular geometry for the C2H5OH (Ethanol) including a description of the C2H5OH bond angles. Lewis Structure for C2H5OH: https://yout.

Is Ethanol (C2H5OH) Polar or Nonpolar? Techiescientist
The Lewis structure is a simple yet powerful tool for understanding the bonding and arrangement of atoms in a molecule. In ethanol, we have a combination of carbon (C), hydrogen (H), and oxygen (O) atoms. To construct the Lewis structure, we start by counting the valence electrons. Carbon has 4 valence electrons, oxygen has 6, and hydrogen has 1.
Ethanol (C2H5OH, EtOH) Fisher Scientific
Hello Guys!In this video, we will determine the Lewis Structure of Ethanol. It has a chemical formula of C2H5OH. To find out its Lewis Structure, we first ca.