Electron Configuration
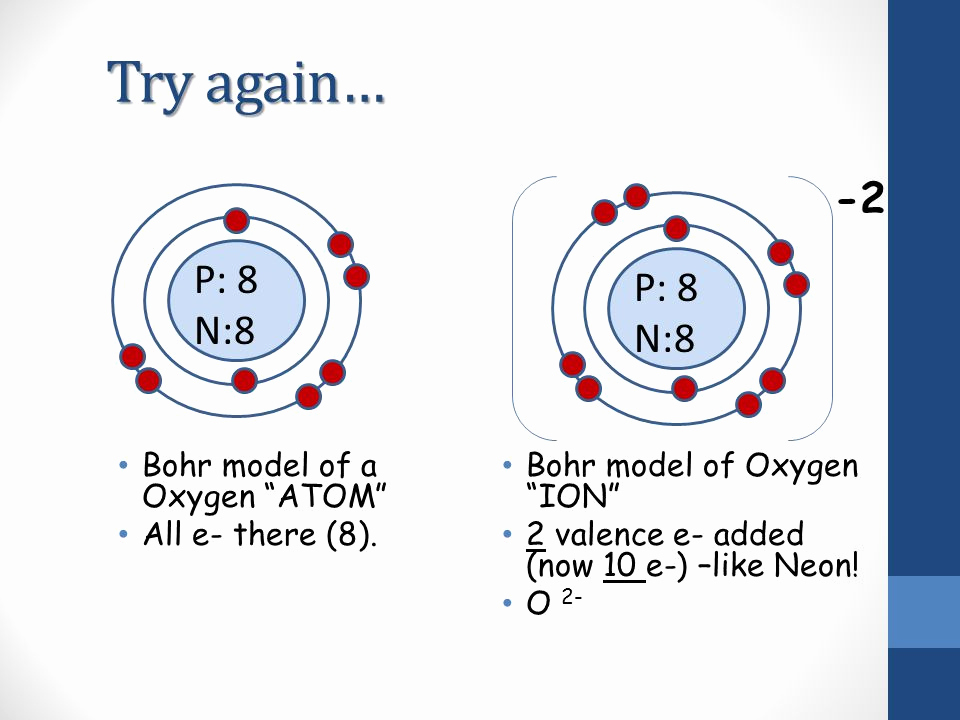
Bohr-Rutherford Diagrams of Ions Positive and Negative Ions When elements form compounds, changes occur in the arrangement of electrons in the outer orbit. Electrons are gained or lost so that element can have a stable electron arrangement of the closest noble gas. Atoms prefer a completely filled outer shell with electrons
Lesson 4 THE STRUCTURE OF THE ATOM WillowWood Lessons
Bohr-Rutherford diagrams are simple atomic models that show the number of electrons in each shell of an atom. While they are a major simplification of what really happening in an atom, they can be useful to help with visualizing electrons orbiting a nucleus. Drawing Bohr-Rutherford diagrams is super easy using the following steps:
Bohr Rutherford Diagrams and ions YouTube
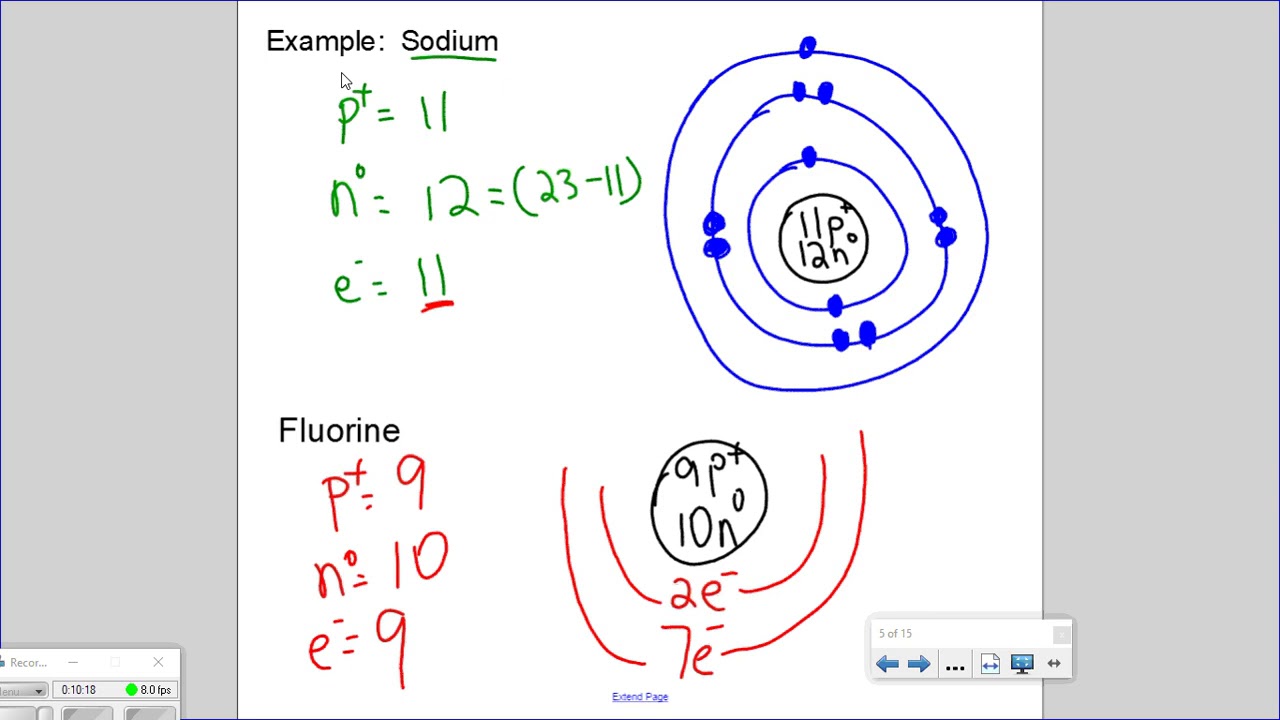
Bohr diagrams for Ionshttp://www.BCLearningNetwork.com.0:01the0:02in0:04this video shows you how to drop or diagrams for Alliance0:08chilling protons neutron.
BohrRutherford diagrams for atoms
In atomic physics, the Bohr model or Rutherford-Bohr model of the atom, presented by Niels Bohr and Ernest Rutherford in 1913, consists of a small, dense nucleus surrounded by orbiting electrons.
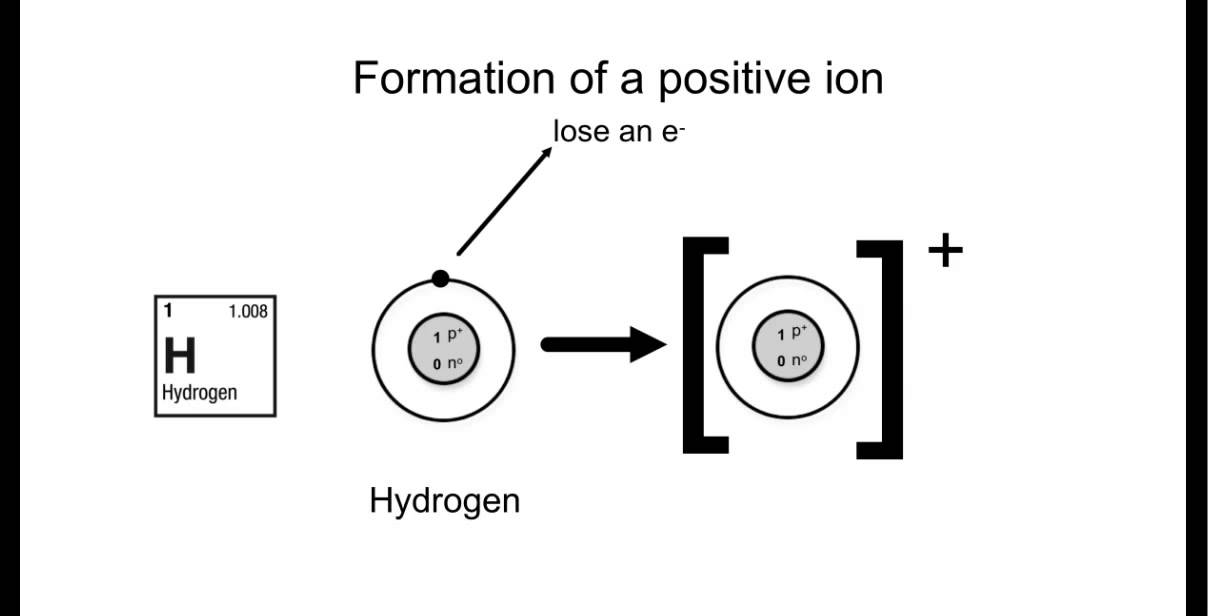
[DIAGRAM] Bohr Diagram For Ion
The plum pudding model was suggested as the first atomic model by J.J Thomson where he suggested that the atom was a sea of positive charge that surrounded small negative electrons. Ernest Rutherford. Ernest Rutherford was a British physicist who by experimenting with gold foil and alpha particles found that there was a large central mass at.

How To Draw Bohr Models For Ions
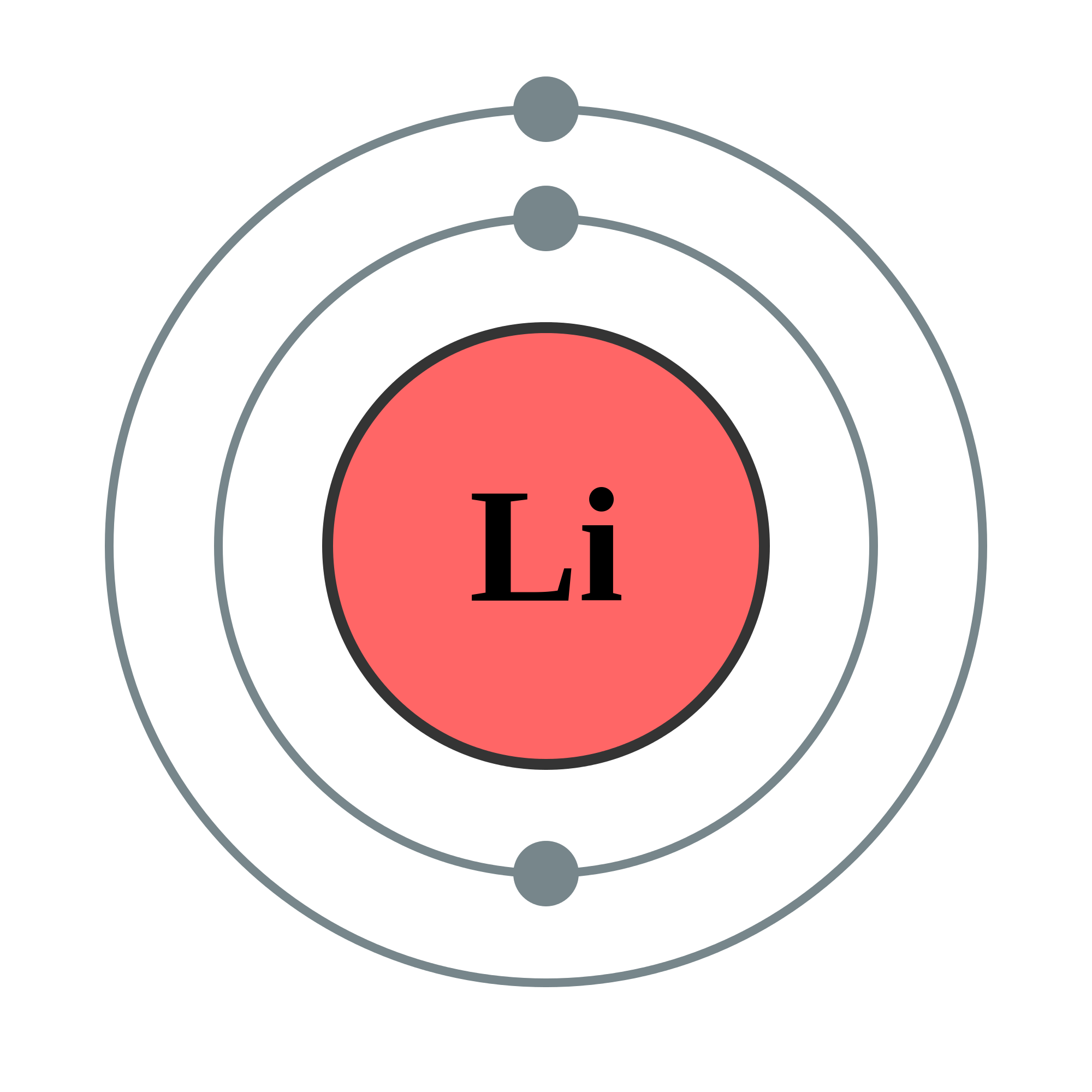
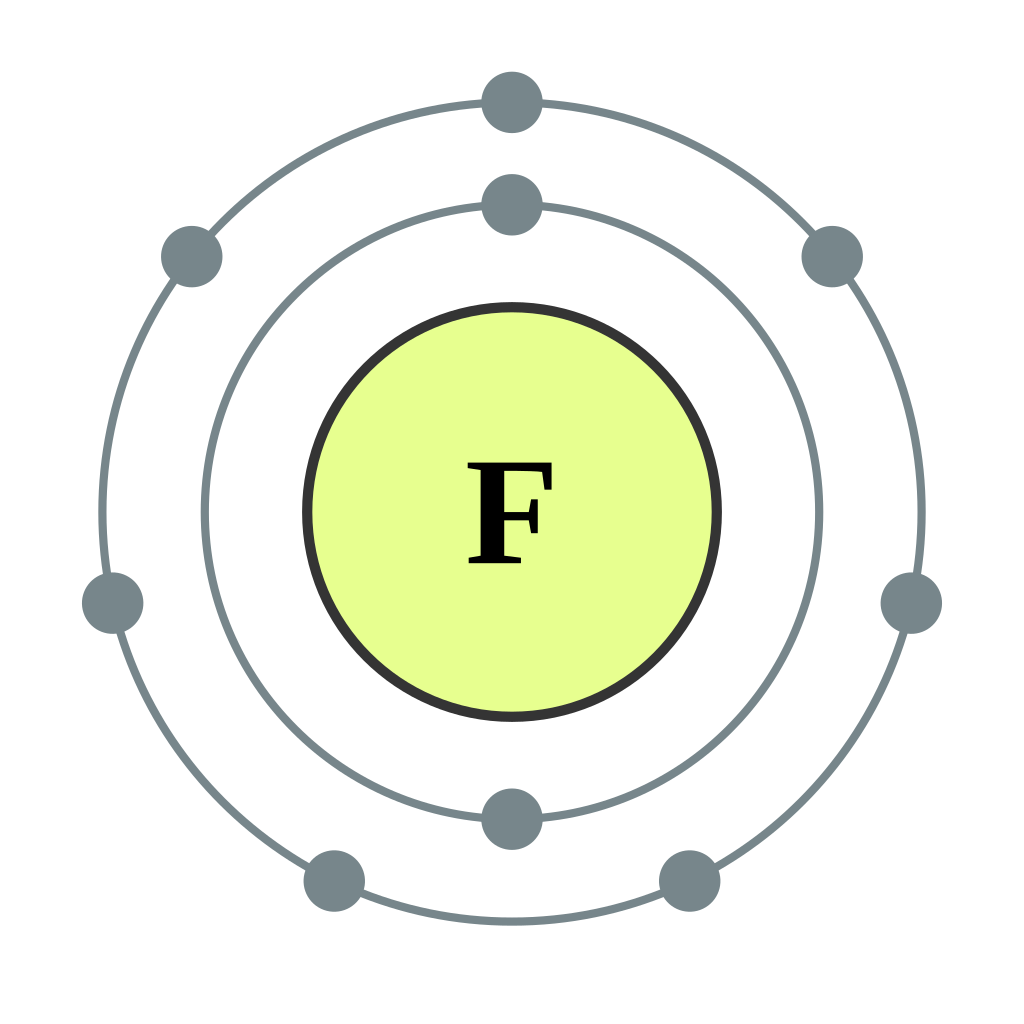
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is.
Bohr Rutherford Diagram For First 20 Elements Wiring Diagram
Since Bohr's model involved only a single electron, it could also be applied to the single electron ions He +, Li 2 +, Be 3 +, and so forth, which differ from hydrogen only in their nuclear charges, and so one-electron atoms and ions are collectively referred to as hydrogen-like or hydrogenic atoms. The energy expression for hydrogen-like.
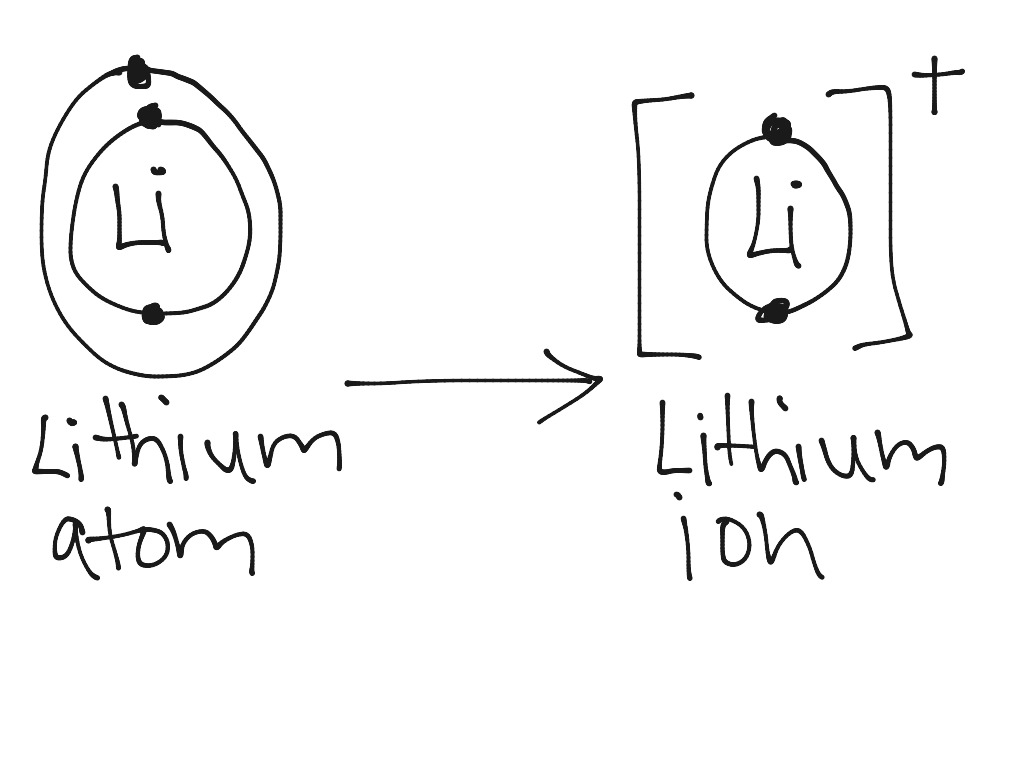
Lithium atom to lithium ion Science, Chemistry, Chemical Bonds ShowMe
Steps to Draw the Bohr Model of Oxygen. The oxygen atom belongs to the 16 th group of the periodic table. The information that we can derive from the above-mentioned Oxygen box is as follows: • The atomic number of Oxygen is 8. • The electronic configuration of Oxygen is [He] 2s 2 2p 4. • The chemical symbol of Oxygen is O.
bohr rutherford diagrams lithium
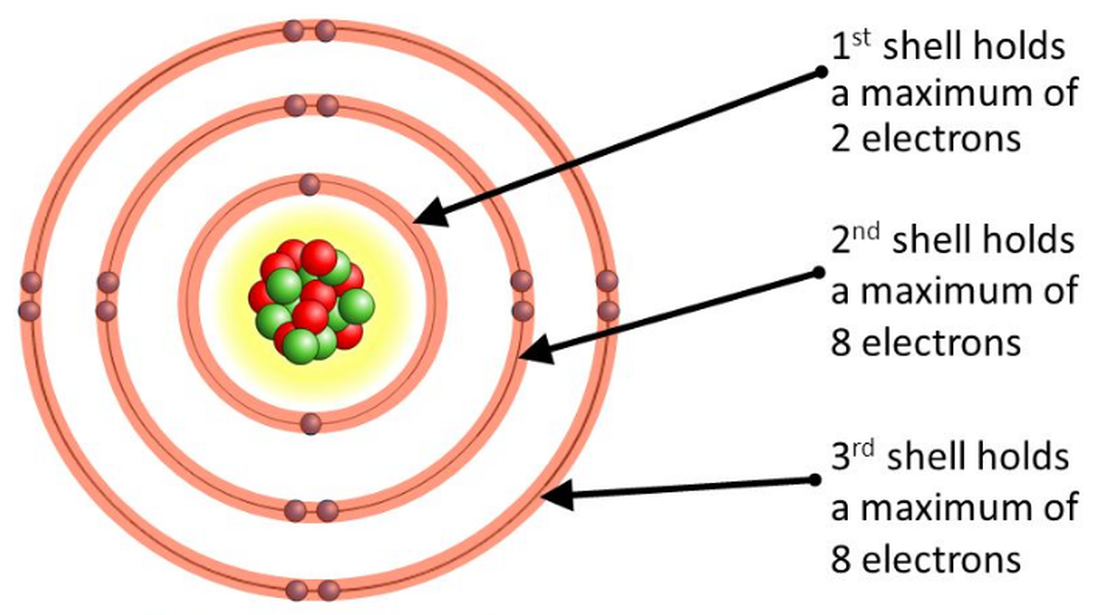
Niels Bohr and Ernest Rutherford studied it and found the number of electrons each orbit needed to maintain the balance between the forces. They drew a diagram to represent the electrons in each orbit. Each orbit in the atom is called an energy shell or energy level. The shell nearest to the nucleus can hold a maximum of 2 electrons.

Image result for silicon atomic model
Resources. Lecture Slides (PDF - 9.3MB) Periodic Table and Table of Constants. Lecture Summary. Prof. Sadoway talks about the principles of modern chemistry and how that led to the understanding of the structure of the atom.He details Bohr's postulates for the hydrogen atom and discusses how the Planck-Einstein relationship applies to electron transitions.
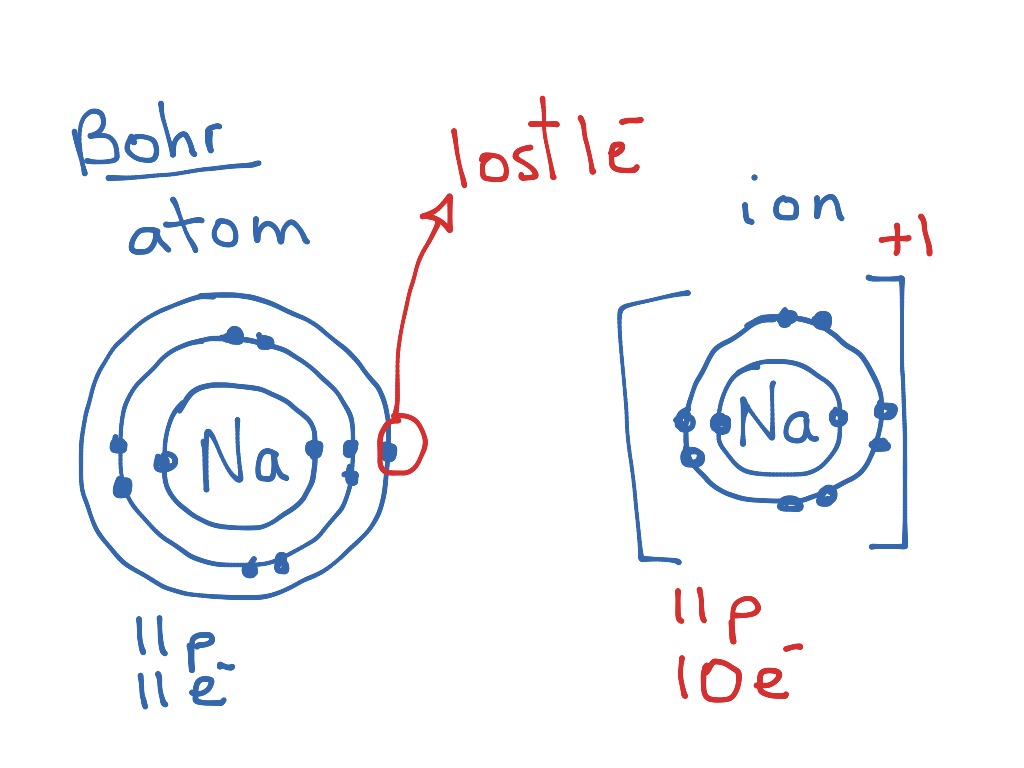
[DIAGRAM] Bohr Diagram For Ion
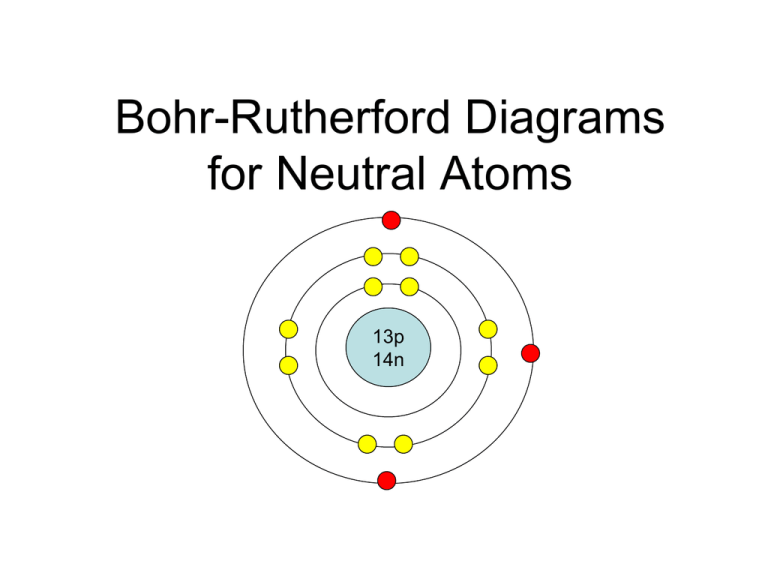
Bohr's model of the atom can be combined with Rutherford's model in diagrams that summarize the numbers and positions of all three subatomic particles. For example, consider the following diagram for Phosphorous: There are certain rules to follow when drawing these diagrams: A circle is drawn in the center to represent the nucleus of the atom.

Potassium Bohr Model Diagram, Steps To Draw Techiescientist
Using the Bohr model, determine the energy, in electron volts, of the photon produced when an electron in a hydrogen atom moves from the orbit with n = 5 to the orbit with n = 2. Show your calculations. Using the Bohr model, determine the lowest possible energy, in joules, for the electron in the Li 2+ ion.
_0.jpg?itok=RuBFwyf1)
Le modèle atomique de RutherfordBohr Alloprof
Here are a few examples of Bohr Rutherford diagrams for different elements: 1. Hydrogen (H) Hydrogen has one electron. In the Bohr Rutherford diagram, the nucleus is represented as a small dot in the center, and the electron is shown in the first energy level surrounding the nucleus. It is represented by a single dot or circle. 2. Oxygen (O)

Bohr's Atomic Model — Overview & Importance Expii
Nitrogen has 2 electrons in its first shell and 5 in its second.Check me out: http://www.chemistnate.com
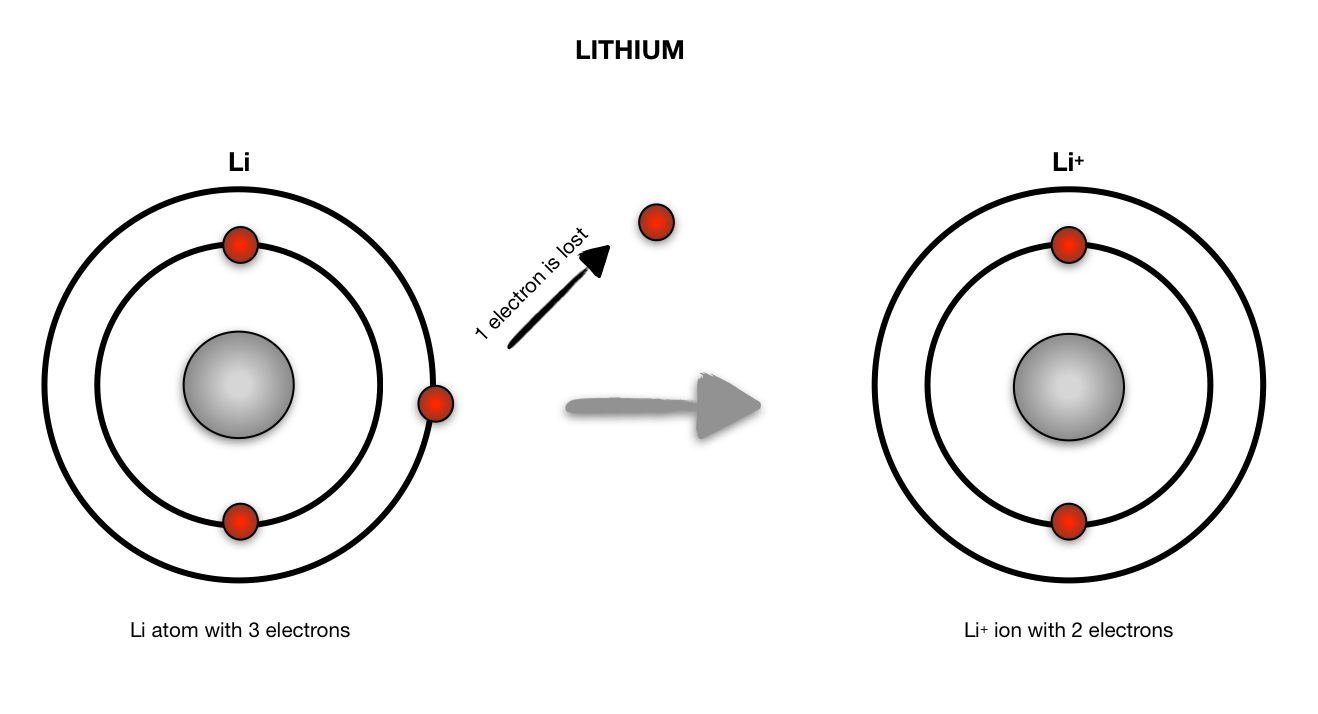
Give an example of an atom and an ion, and draw a BohrRuthe Quizlet
0:00 / 5:40 Bohr-Rutherford Diagram of NaCl (sodium chloride, table salt) chemistNATE 260K subscribers Subscribe Subscribed Share 6.7K views 3 years ago NaCl, sodium chloride, is an IONIC.
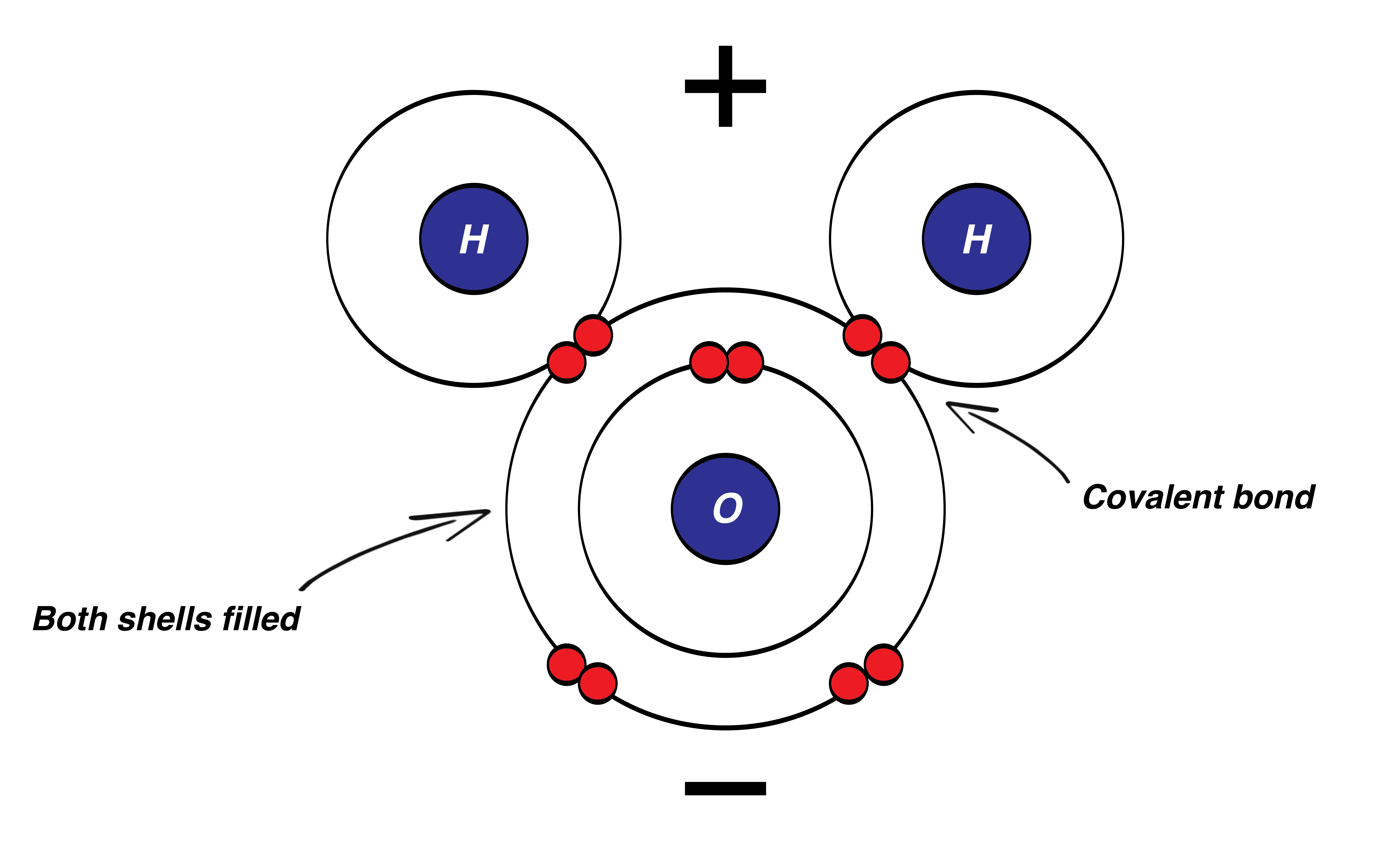
Bohr Rutherford Diagram For Nitrogen
Manish Bhardwaj. 5.6: Bohr Diagrams of Atoms and Ions is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells,..