Chem Filling in the Valence Electrons of an Electron Dot Structure
A step-by-step explanation of how to draw the H3O+ Lewis Dot Structure (Hydronium ion).For the H3O+ structure use the periodic table to find the total number.

A stepbystep explanation of how to draw the H3O+ Lewis Structure
Figure 15.4.3 15.4. 3: The ammonium ion. When drawing the Lewis structure of a polyatomic ion, the charge of the ion is reflected in the number of total valence electrons in the structure. In the case of the ammonium ion: 1 N 1 N atom = 5 = 5 valence electrons. 4H 4 H atoms = 4 × 1 = 4 = 4 × 1 = 4 valence electrons.
Lewis Dot Structure For H3o Science Chemistry Showme
Hydronium is the positive ion present in Arrhenius acid solutions. It is formed from a hydrogen ion and water bonding. Hydronium contains 2 polar covalent bonds and 1 coordinate covalent bond. Lewis Dot Structure of H3O+, (Hydronium Ion) from http://treefrog.fullerton.edu/chem/LS/H3OplusLS.html Chemical Demonstration Videos

Lewis Dot Structure For H3o Science Chemistry Showme
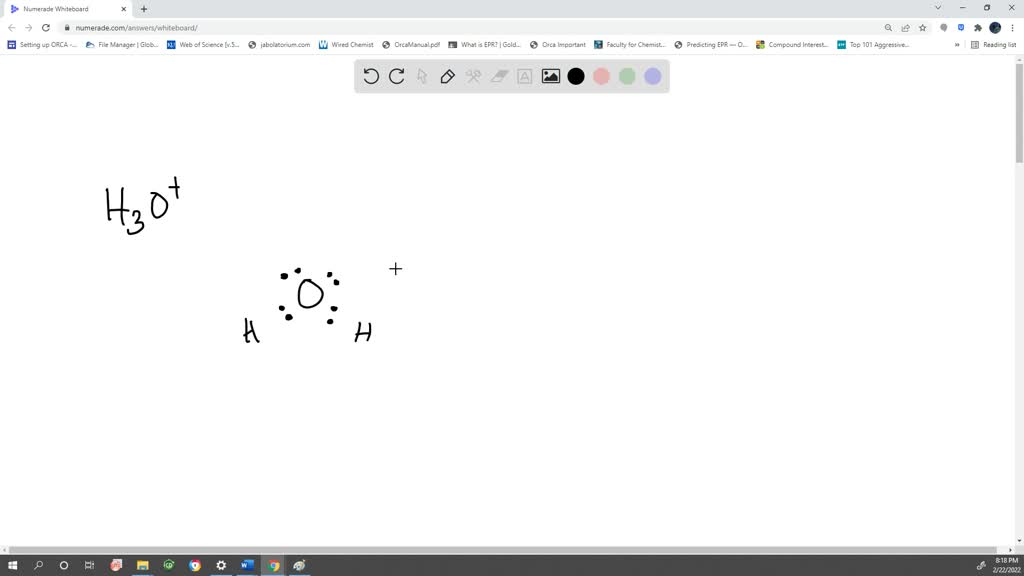
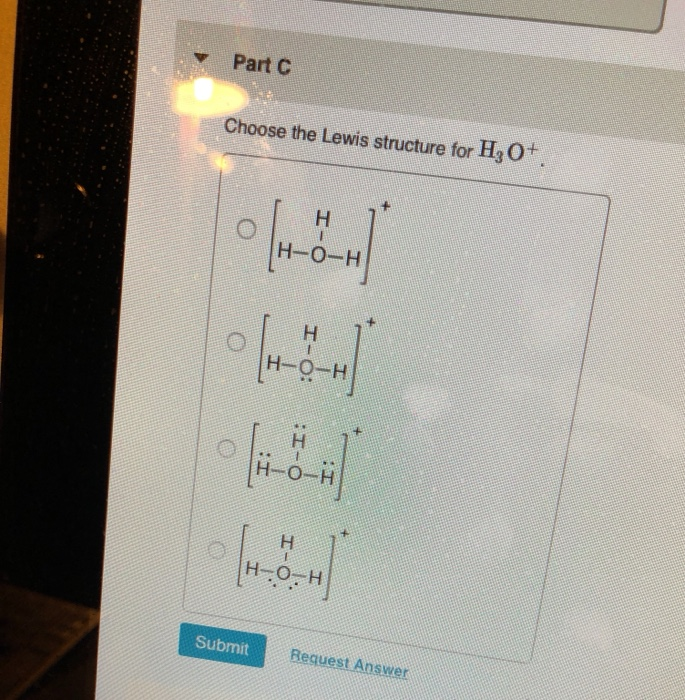
Lewis structure of H3O+ ion (Hydronium ion) contains three single bonds between the Oxygen (O) atom and each Hydrogen (H) atom. The Oxygen atom (O) is at the center and it is surrounded by 3 Hydrogen atoms (H). The Oxygen atom has one lone pair and it also has +1 formal charge. Let's draw and understand this lewis dot structure step by step.
Lewis Dot Structure for H3O^+ Science, Chemistry ShowMe
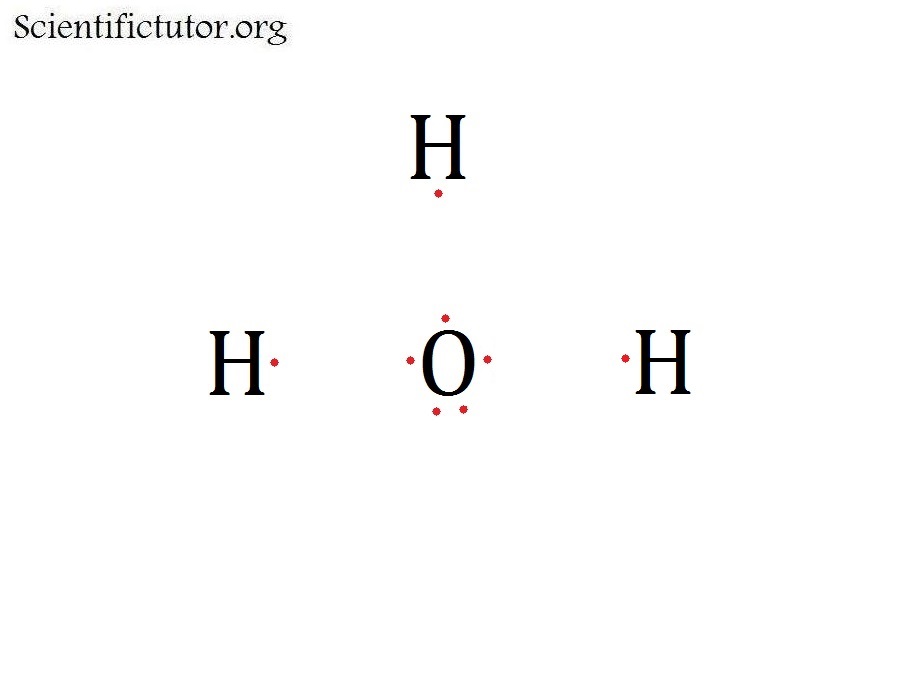
We can illustrate the formation of a water molecule from two hydrogen atoms and an oxygen atom using Lewis dot symbols: The structure on the right is the Lewis electron structure, or Lewis structure, for H 2 O. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Moreover, by sharing a bonding pair with oxygen.
Media Portfolio
The most stable Lewis dot structure of the H3O+ ion is because the hydronium ion only has a +1 formal charge, the lowest one. H3o+ lewis structure angle. The angle that the central atom forms with the bonded atom are referred to as the bond angle. Due to the attraction of electron density regions surrounding the central atoms, the Bond angle.
SOLVED Draw an electrondot structure for the hydronium ion, H3O , and
Lewis Structures. Page ID. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the "best" electron.

H3O+ Lewis Structure, Geometry, Hybridization, and MO Diagram
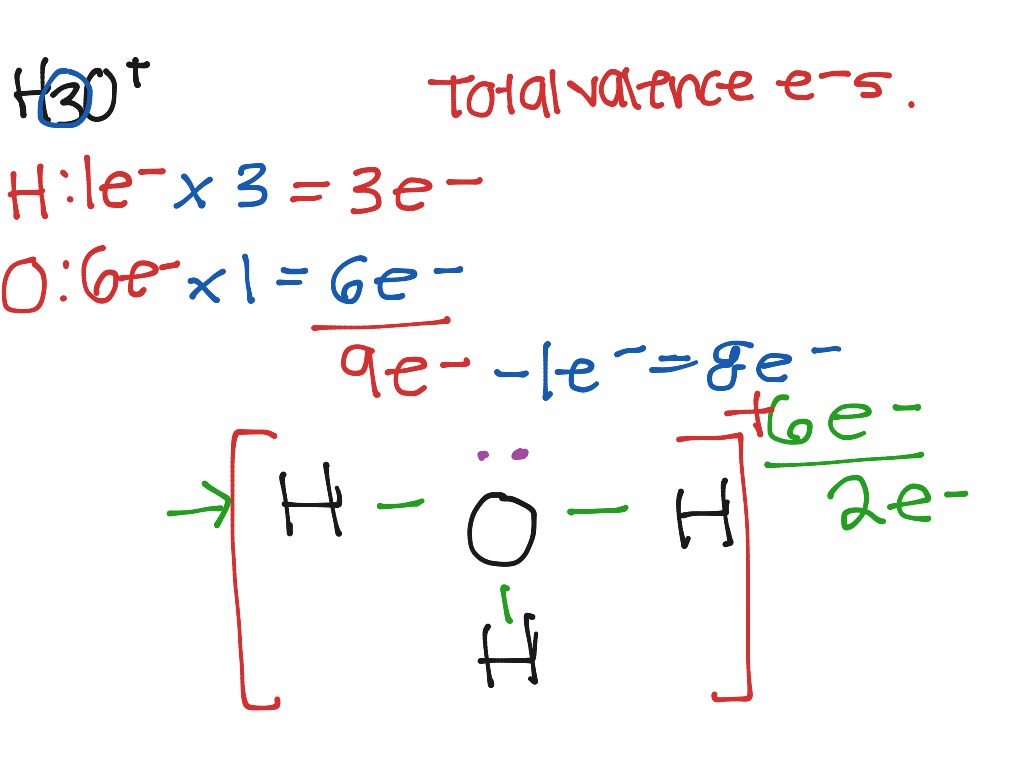
Let's do the Lewis structure for H3O+, the hydronium ion. On the periodic table, Hydrogen's in group 1, 1 valence electron; but we have 3 Hydrogens. Plus Oxygen, group 6 or 16; 6 valence electrons. And this plus sign means we've lost a valence electron, we've lost a negative charge. So we actually need to subtract 1.

19. Lewis Dot Structure of H3O+ How to Draw Lewis Structures Class
Center atom selection Mark lone pairs on atoms Mark charges on atoms if there are charges. Check the stability and minimize charges on atoms by converting lone pairs to bonds to obtain best lewis structure. Total number of electrons of the valance shells of H 3 O + There are two elements in hydronium ion; hydrogen and oxygen.

H3O+ Lewis Structure Lewis Dot Structure for H3O+ Hydronium ion
Hydrogen = 1 3*Hydrogen = 3 Oxygen = 6 Total = 9 Now the important point is, not to forget about the + sign. + sign indicates losing an electron from the total valence electrons. ( - sign indicates gaining an electron) Thus, the total valence electron is 8 now.

Draw a Lewis structure for H3O+ . Include all hydrogen atoms and show
1. Count the total valence electrons in [H3O]+ The Lewis dot structure of a molecule is referred to as a simplified representation of all the valence electrons present in it. Therefore, the very first step while drawing the Lewis structure of [H 3 O] + is to find the total valence electrons present in the concerned elemental atoms.

Lewis Structure of Hydronium H3O+ YouTube
A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 7.9 Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table.
H3o Lewis Dot Structure
A video explanation of how to draw the Lewis Dot Structure for the Hydronium Ion, along with information about the compound including Formal Charges, Polarit.

H3O+ Lewis Structure (Hydronium Ion) YouTube
Step 1 Basic concepts:- Lewis structure :- The lewis structure is a representation of velance electron in a m. View the full answer Step 2 Unlock Step 3 Unlock Answer Unlock Previous question Next question Transcribed image text: Draw the Lewis structure for the (H3O+) ion. Not the question you're looking for?

The Lewis Dot Structure Is Also Called The Electron Dot Diagram
Steps of drawing H3O+ lewis structure Step 1: Find the total valence electrons in H3O+ ion In order to find the total valence electrons in H3O+ ion, first of all you should know the valence electrons present in oxygen atom as well as hydrogen atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.)
Lewis Dot Structure For H3o Science Chemistry Showme
Lewis Dot Structure of H3O+, (Hydronium Ion) kentchemistry.com 25.1K subscribers Subscribe Subscribed 415 160K views 12 years ago Every Video I quickly take you through how to draw the.