Illustrated Glossary of Organic Chemistry Distillation (simple distillation, fractional
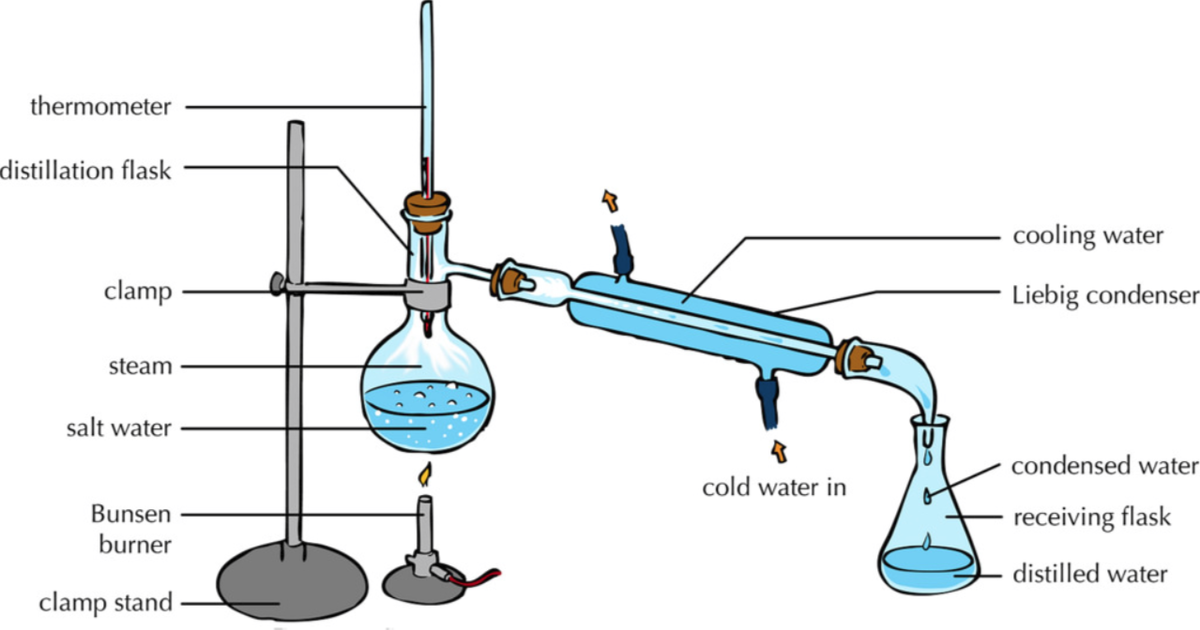
The distillation apparatus, commonly called a 'still', consists of a vessel for plant material and water, a condenser to cool and condense the vapour produced and a method of collection, or 'receiver'.Material from the appropriate part of the plant for extraction is immersed in water in the distillation vessel. This is then heated to boiling point and the steam (water vapour) carries.
Distillation What Is Distillation? Process and Uses of Distillation
An apparatus using a steam line is shown in Figure 5.59. It is assumed that readers have previously performed a simple distillation, so in this section are described differences between simple and steam distillations. Figure 5.58: Steam distillation of orange peel using water in the distilling flask and a Bunsen burner.
Distillation Key Stage Wiki
Fractional distillation is often used to separate mixtures of liquids that have similar boiling points. It involves several vaporization-condensation steps (which takes place in a fractioning column). This process is also known as rectification. The apparatus required to perform a fractional distillation on a mixture is listed below.

chemistry Students Britannica Kids Homework Help
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize.It uses distillation to fractionate.Generally the component parts have boiling points that differ by less than 25 °C (45 °F) from each other under a pressure of one atmosphere.
Distillation Apparatus, 24/40 joints
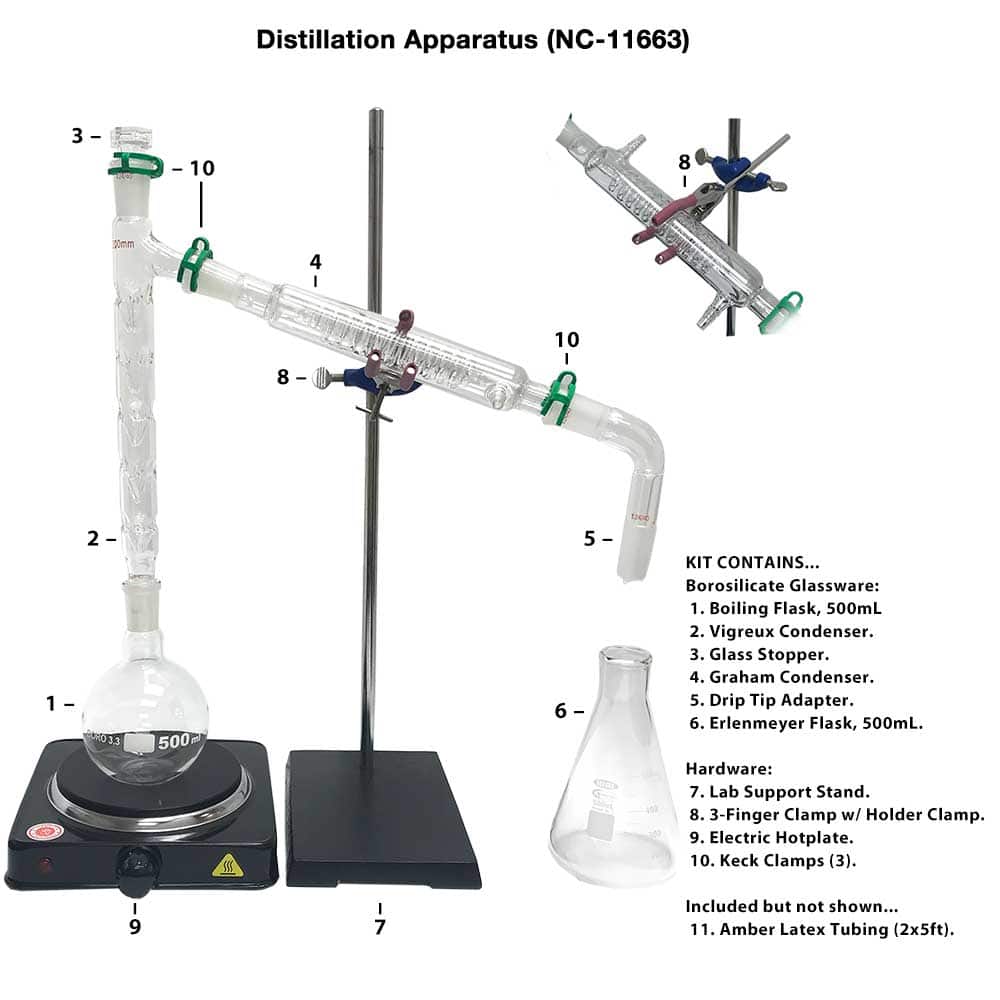
use apparatus to carry out reflux and adapt the apparatus for distillation, setting up the equipment using a variety of glassware, including Quickfit®, retort stands and clamps; Republic of Ireland. Junior Cycle. Science. Chemical world. Building blocks. 2.
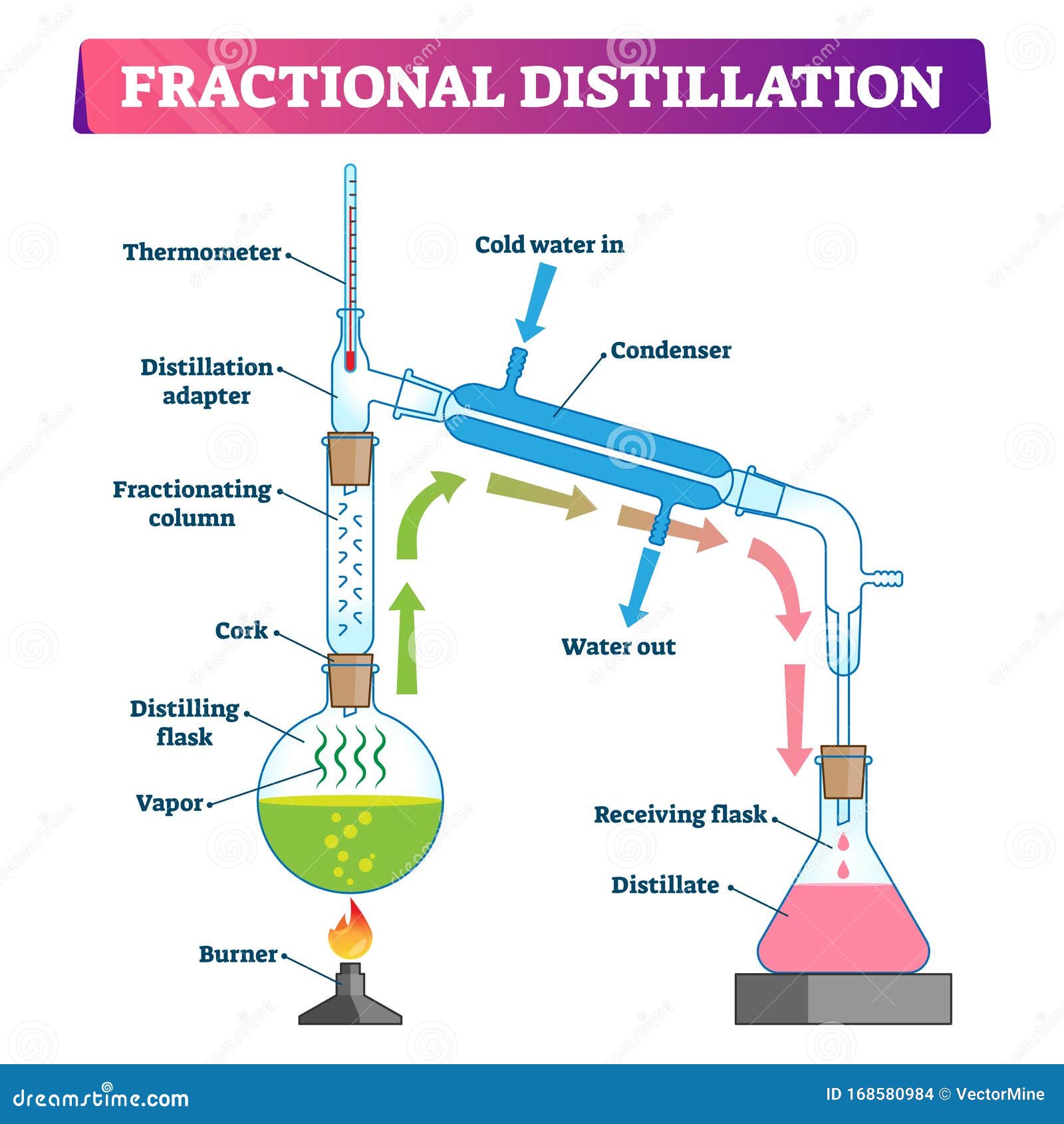
Fractional Distillation Vector Illustration. Labeled Educational Process. Stock Vector
It is assumed that readers have previously performed a simple distillation, so in this section are described differences between simple and fractional distillation. Figure 5.43: Fractional distillation apparatus. Figure 5.44: a) Removal of glass wool plug on a beaded fractionating column, b) Insulating the column with foil, c+d) Condensation on.
/chemistry-distillation-58aef7d15f9b58a3c92007fa.jpg)
What Is Distillation? Principles and Uses
Learning Objectives. Make sure you thoroughly understand the following essential ideas: Sketch out a typical boiling point diagram for a binary liquid solution, and use this to show how a simple one-stage distillation works.; Explain the role of the lever rule in fractional distillation; Describe the purpose and function of a fractionating column; Sketch out boiling point diagrams for high.

With the neat labelled diagram, explain the process of distillation. Brainly.in
Vacuum Distillation Procedure. A vacuum distillation apparatus is shown in Figure 5.50, using a simple distillation setup. A fraction distillation can also be used. It is assumed that readers have previously performed a simple distillation under atmospheric pressure, so in this section are described differences between atmospheric and reduced pressure distillations.

simple distillation Mychem
Distillation is a separation technique is used to remove a solvent from a mixture and keep it rather than it mixing with the air and being lost. Learn more in this KS3 Chemistry guide from Bitesize.
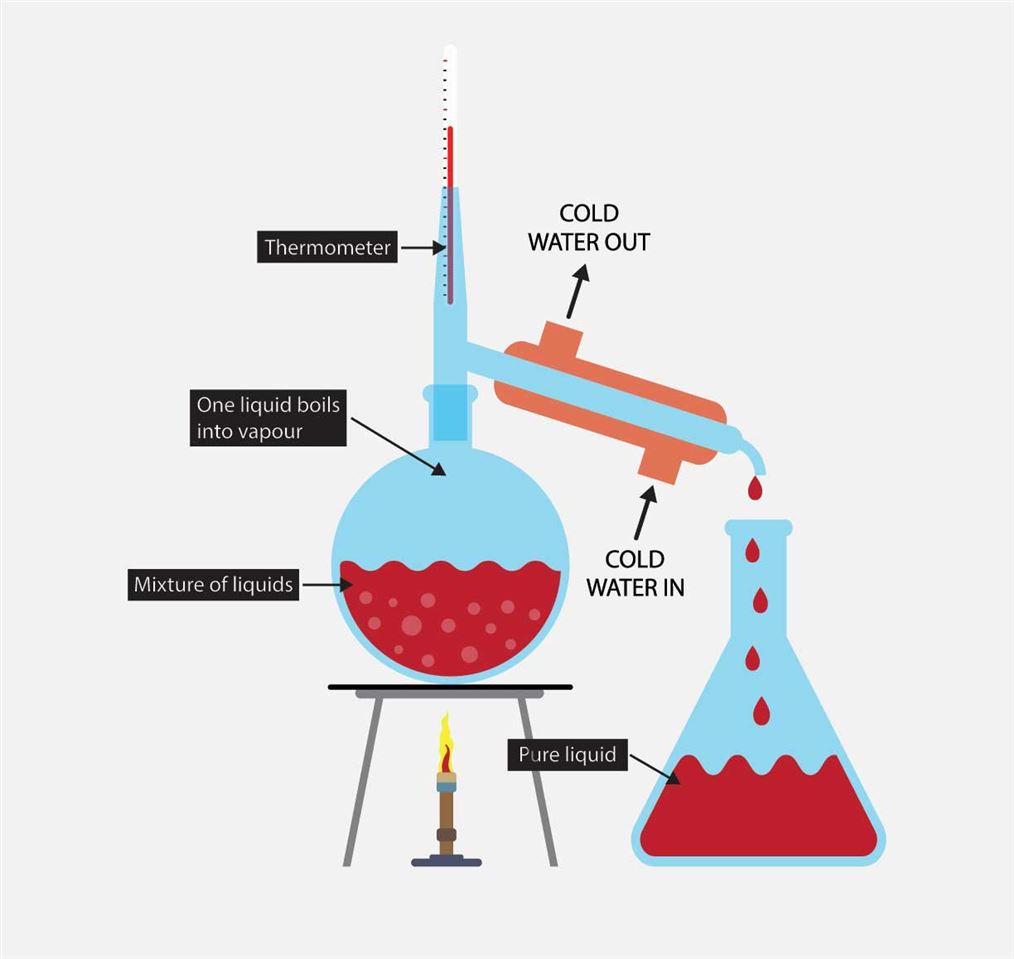
What is Distillation and How is Liquor Made? The People's Bourbon Review
Fractional Distillation Procedure. Few fractional distillation apparatuses are required for the process. It includes distilling flask, condenser, receiver, fractionating column, thermometer and heat source. After setting up the apparatus, a mixture of two miscible liquids A and B is taken where A has more volatility than substance B.
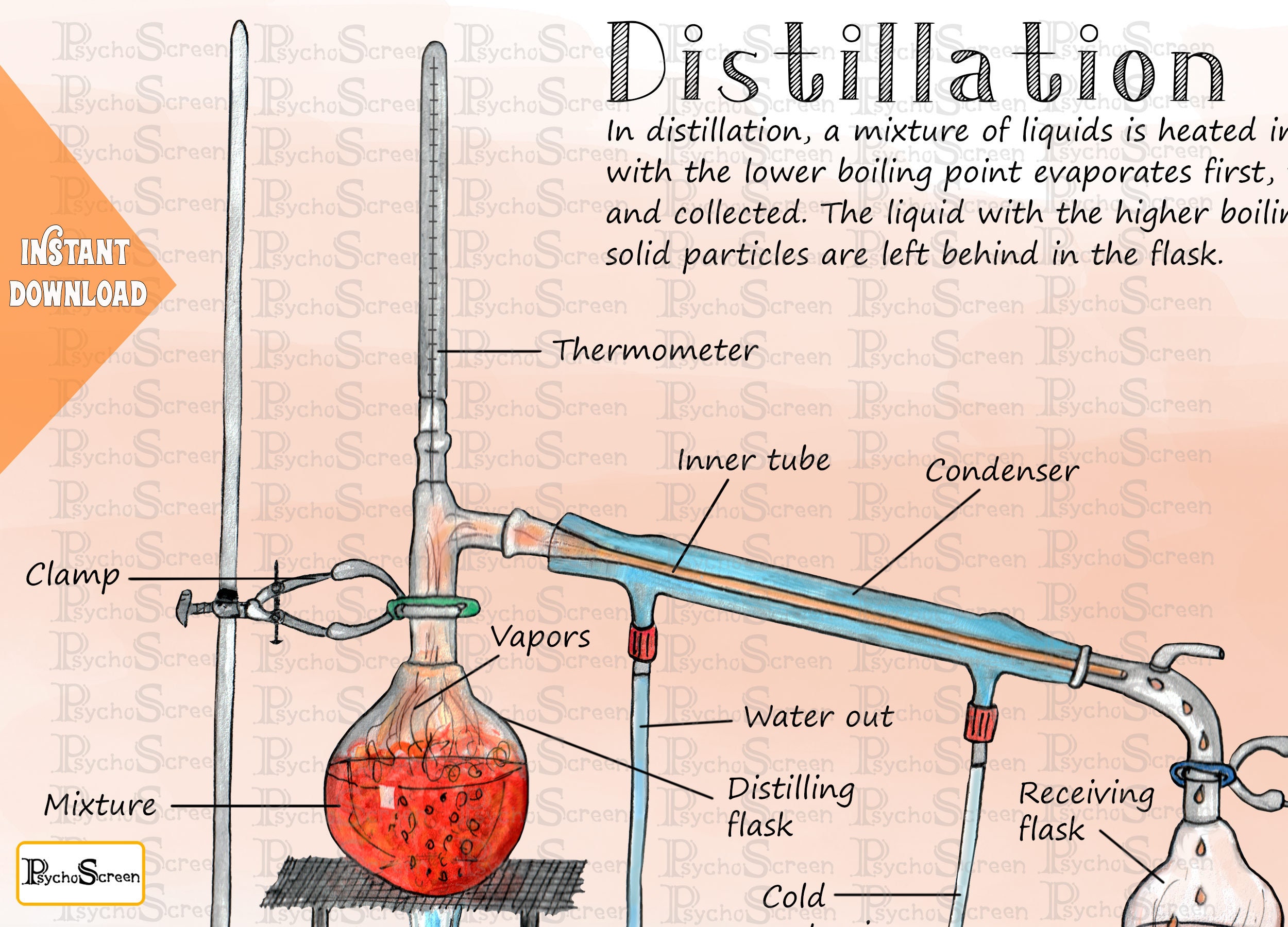
Distillation Apparatus Diagram Poster Printable Lab Tools Etsy
distillation, process involving the conversion of a liquid into vapour that is subsequently condensed back to liquid form. It is exemplified at its simplest when steam from a kettle becomes deposited as drops of distilled water on a cold surface.Distillation is used to separate liquids from nonvolatile solids, as in the separation of alcoholic liquors from fermented materials, or in the.

Distillation process diagram for education 3227893 Vector Art at Vecteezy
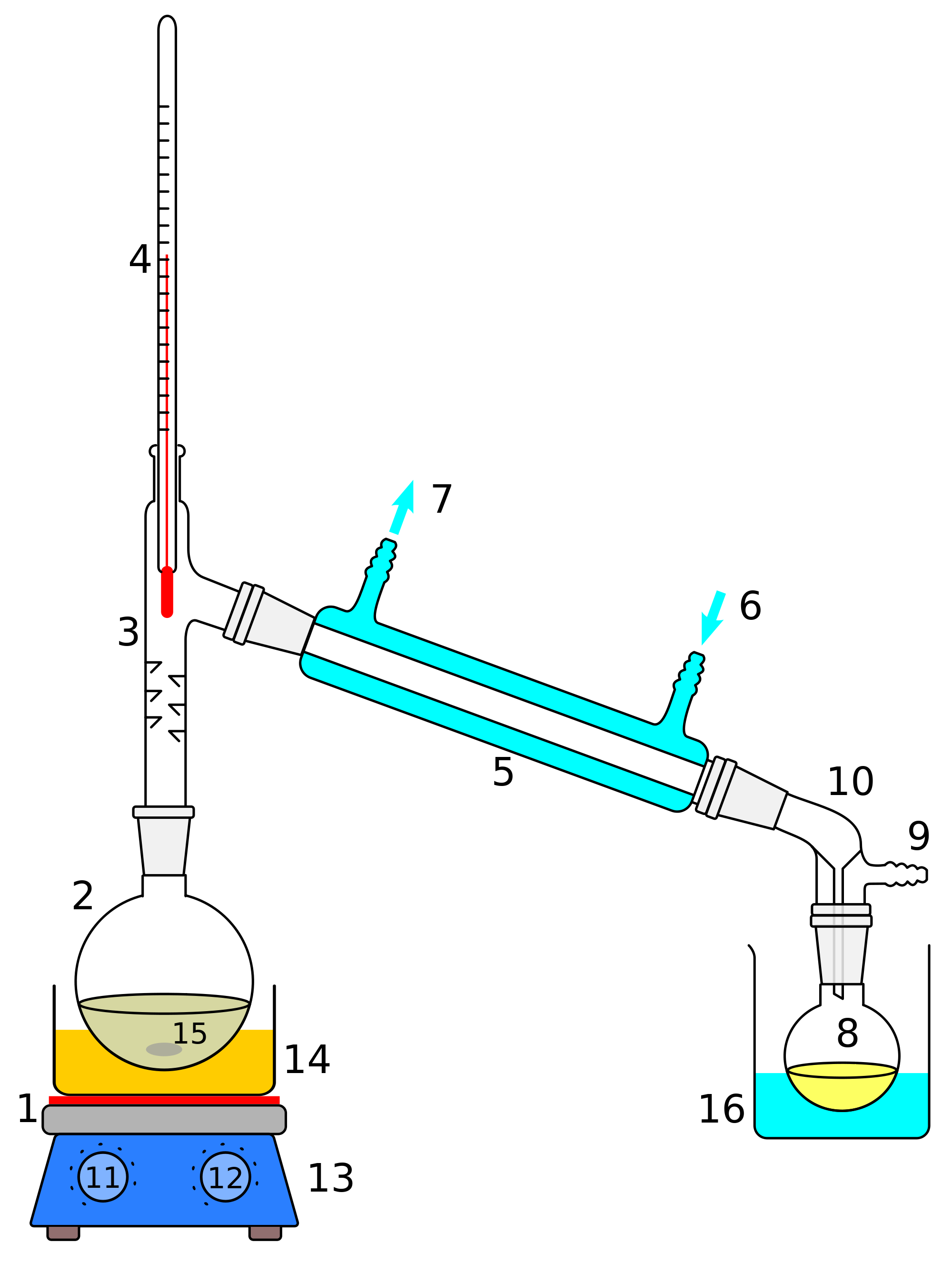
Figure 5.21: a) Distillation apparatus arranged on the benchtop, b) Correct clamping of the round bottomed flask, c) Incorrect clamping. Assemble the Apparatus: To visualize the assembly of the apparatus, it may be helpful to first lay out the glassware on the benchtop before assembling the parts (Figure 5.21a).

Labelled Diagram Of Distillation
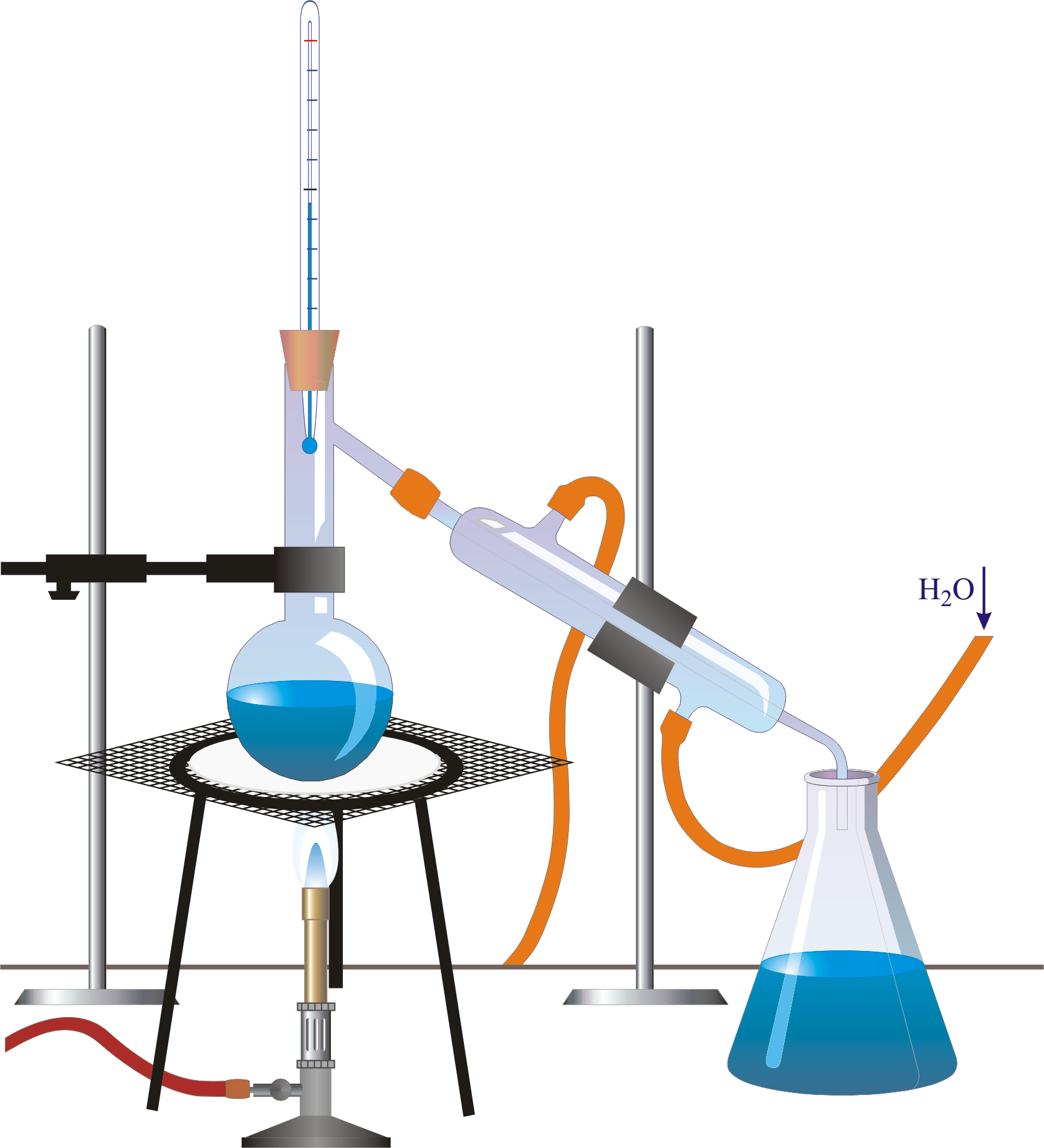
Butte College. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. In a distillation, a liquid is boiled in the "distilling flask," then the vapors travel to another section of the apparatus where they come into contact with a cool surface.
Chemistry Glossary Search results for 'distillation'
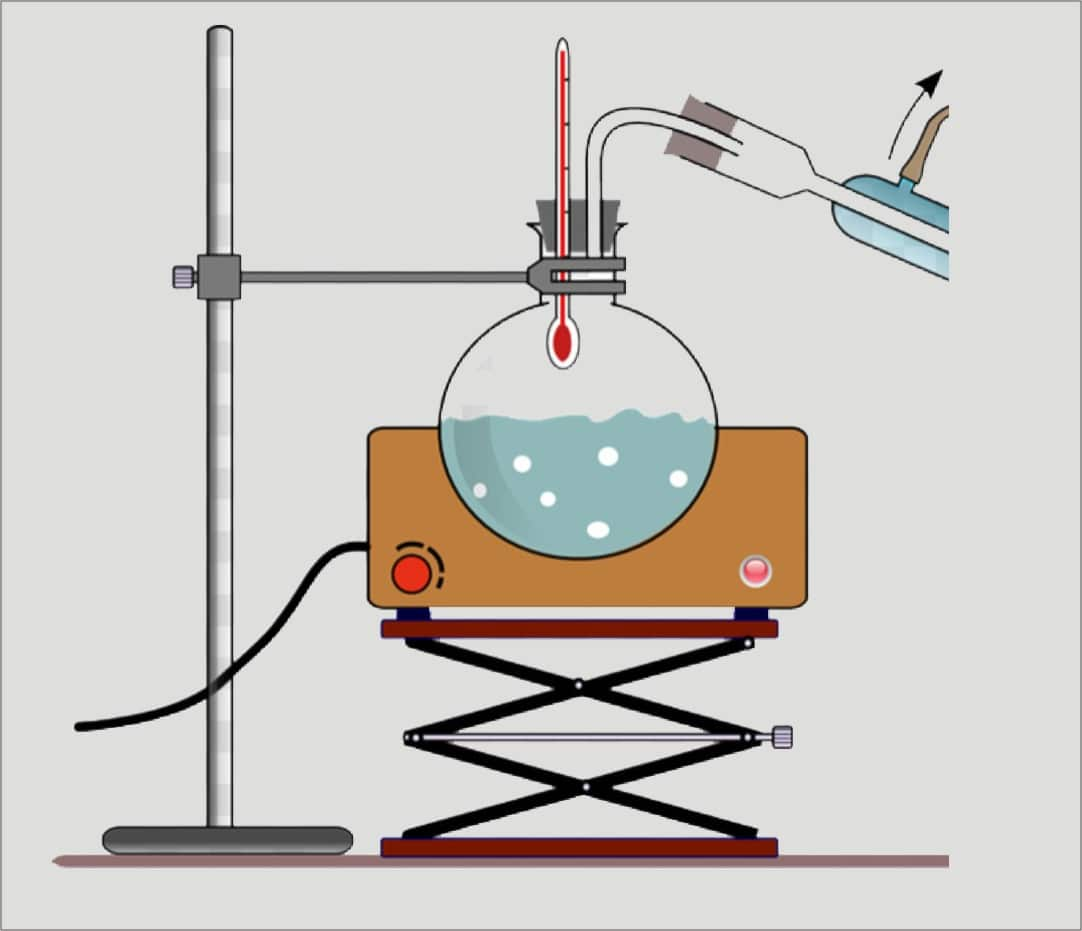
Setting Up the Apparatus. The liquid you are going to distill goes into one beaker, along with a boiling chip. This beaker sits on the hot plate, since this is the liquid you will be heating. Insert a short length of glass tubing into a stopper. Connect it to one end of a length of plastic tubing.
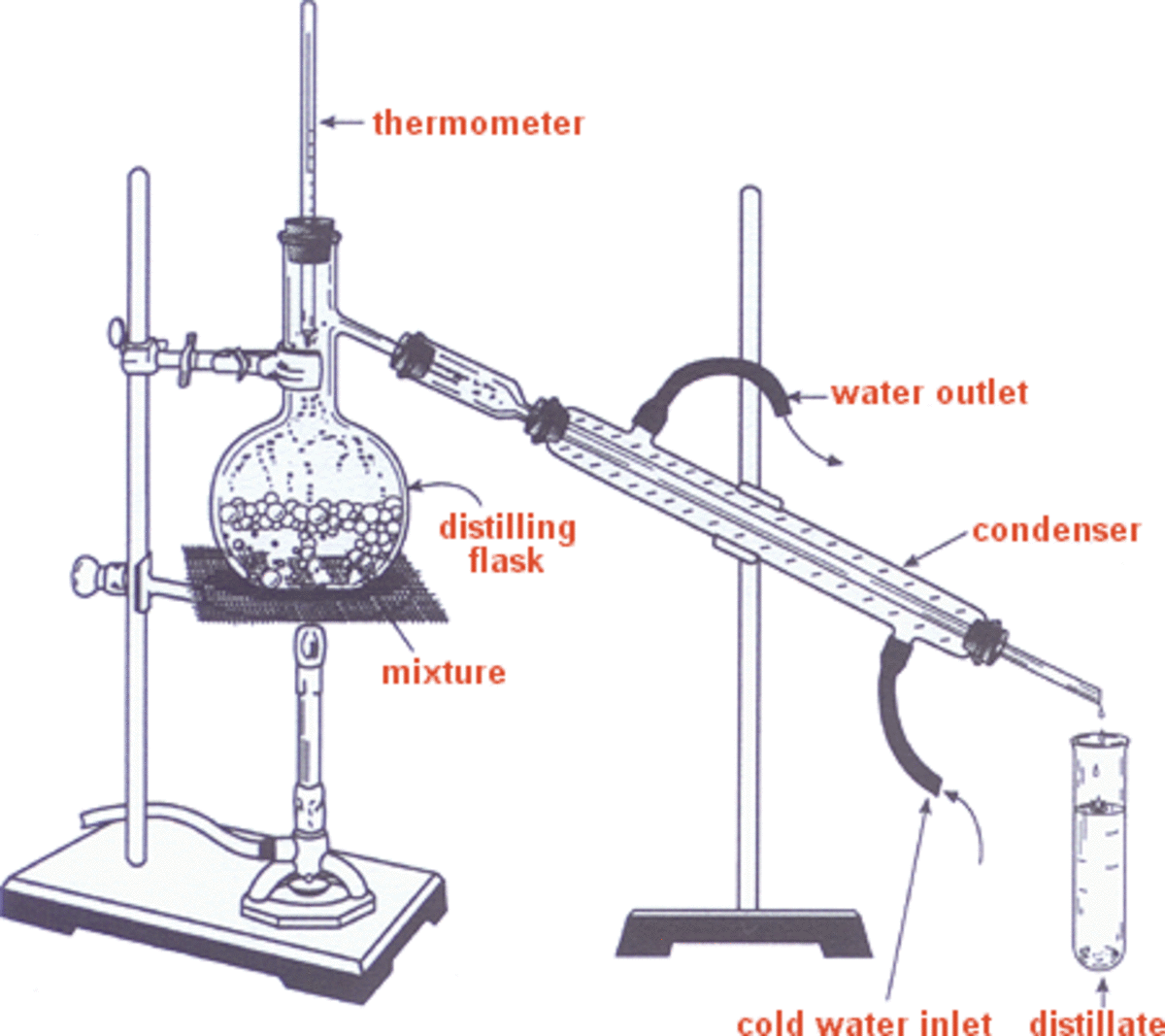
Alcohol Distillation and its Origins HubPages
A completely sealed distillation apparatus could experience extreme and rapidly varying internal pressure, which could cause it to burst open at the joints. Therefore, some path is usually left open (for instance, at the receiving flask) to allow the internal pressure to equalize with atmospheric pressure.. Diagram of a typical industrial.
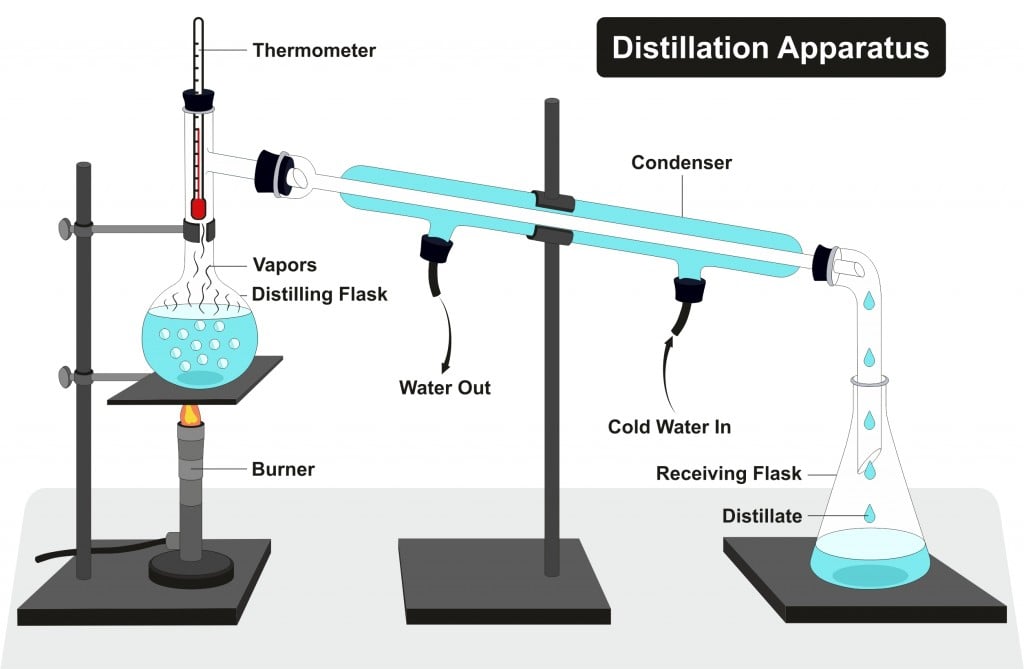
Denatured Alcohol Definition, Properties, Examples And Uses
and the distillation apparatus. Distillation relies on the fact that the vapor above a liquid mixture is richer in the. vapor/liquid diagrams for pairs of solvents. The graph below (Fig. 5) shows such a diagram for 2 solvents, A and B. A is the lower boiling material. The bottom of the graph shows the liquid state and the top of the graph