The Structure Of An Atom Explained With A Labeled Diagram Best Diagram Collection
Because the sum of the numbers of protons and neutrons equals the mass number, 127, the number of neutrons is 74 (127 − 53 = 74). Since the iodine is added as a 1− anion, the number of electrons is 54 [53 - (1-) = 54]. Exercise 2.2.1 2.2. 1. An ion of platinum has a mass number of 195 and contains 74 electrons.
:max_bytes(150000):strip_icc()/atom-drawn-by-scientist-or-student-155287893-584ee6855f9b58a8cd2fc8f1.jpg)
Subatomic Particles You Should Know
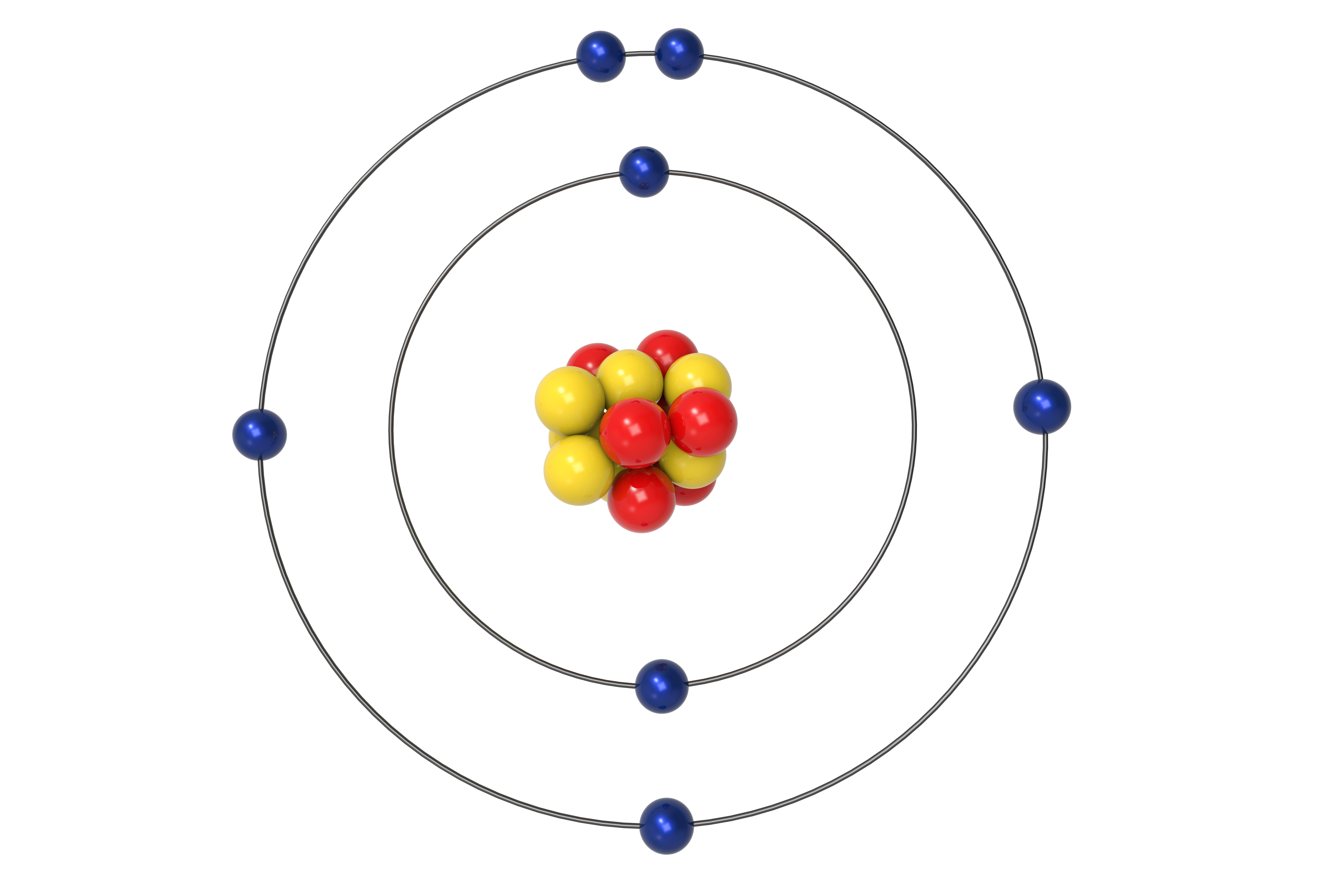
An early model of the atom was developed in 1913 by the Danish scientist Niels Bohr (1885-1962). The Bohr model shows the atom as a central nucleus containing protons and neutrons, with the electrons in circular electron shells at specific distances from the nucleus, similar to planets orbiting around the sun.. 3D diagram of circular 1s.
Structure Of An Atom Structure Of Atom Diagram, HD Png Download kindpng
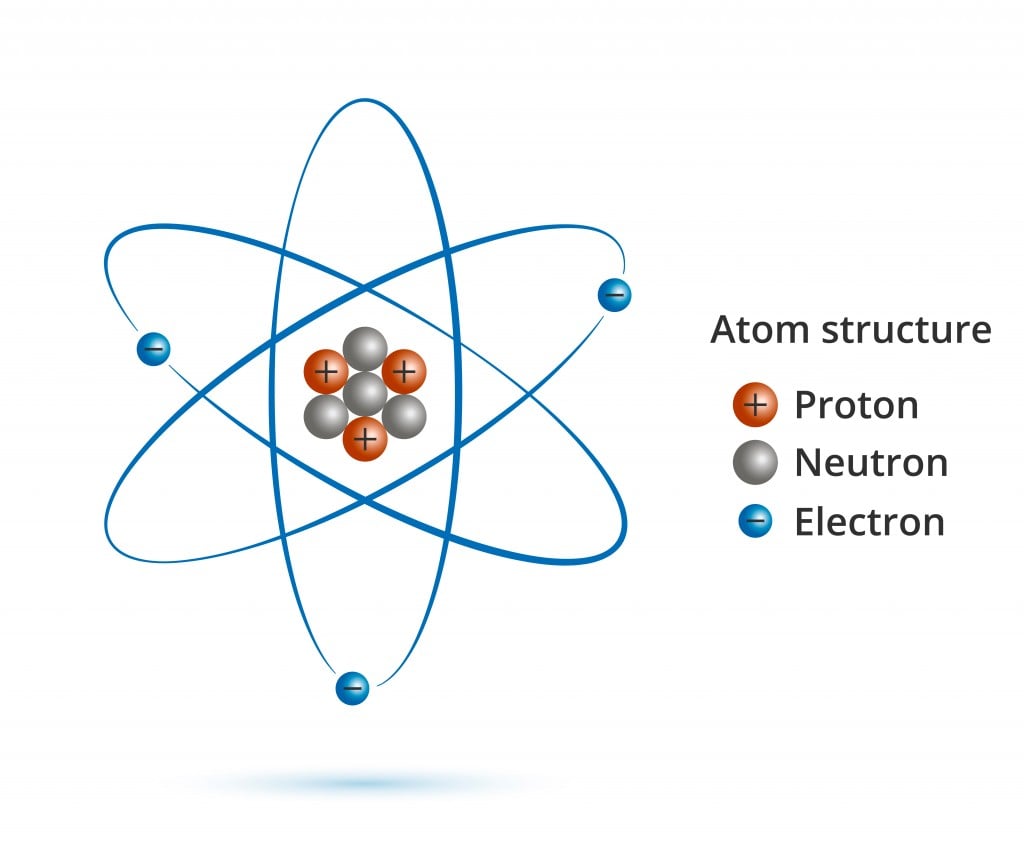
Negative particles in the electron cloud. Discovered by JJ Thomson. Particle with no charge in the nucleus of an atom. Discovered by Chadwick. Positive particles found in the nucleus of an atom. Discovered by Ernest Rutherford. Start studying Label the Atom. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
Atoms and Atomic Structure HubPages
According to the diagram, this helium atom contains two protons, two neutrons, and two electrons. The numbers of protons and electrons make sense: the atomic number of helium is 2 , so any helium atom must have two protons in its nucleus (otherwise, it would be an atom of a different element!).And, because this is a neutral atom, it must contain two electrons to balance out the positive.

Atomic structure Mychem
Physical Chemistry (Essentials) - Class 11 8 units · 52 skills. Unit 1 Welcome to physical chemistry. Unit 2 Structure of atom. Unit 3 Some basic Concepts of Chemistry. Unit 4 Redox reactions. Unit 5 Gaseous state. Unit 6 Thermodynamics. Unit 7 Chemical Equilibrium. Unit 8 Ionic equilibrium.

Atom American Welding Society Education Online
If there are more protons than electrons, an atomic ion has a positive charge and is called a cation. If there are more electrons than protons, the ion has a negative charge and is called an anion. Elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). However, it's easy to determine the configuration of electrons for heavier.

Structure Of An Atom Class 9 Science Notes Leverage Edu
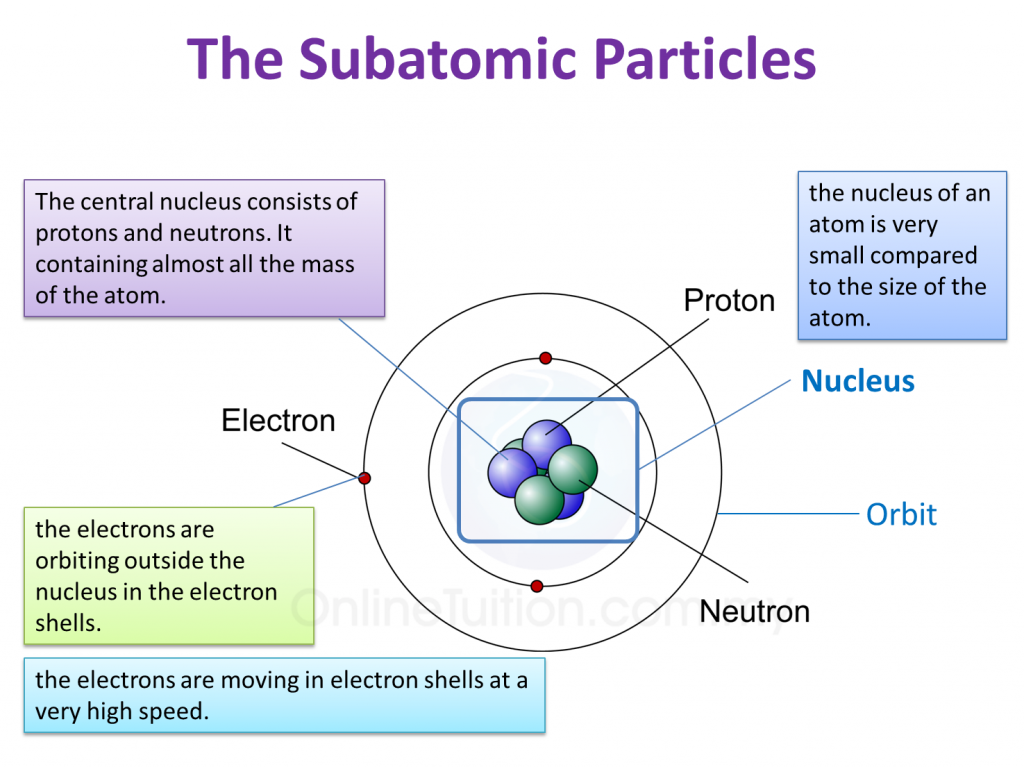
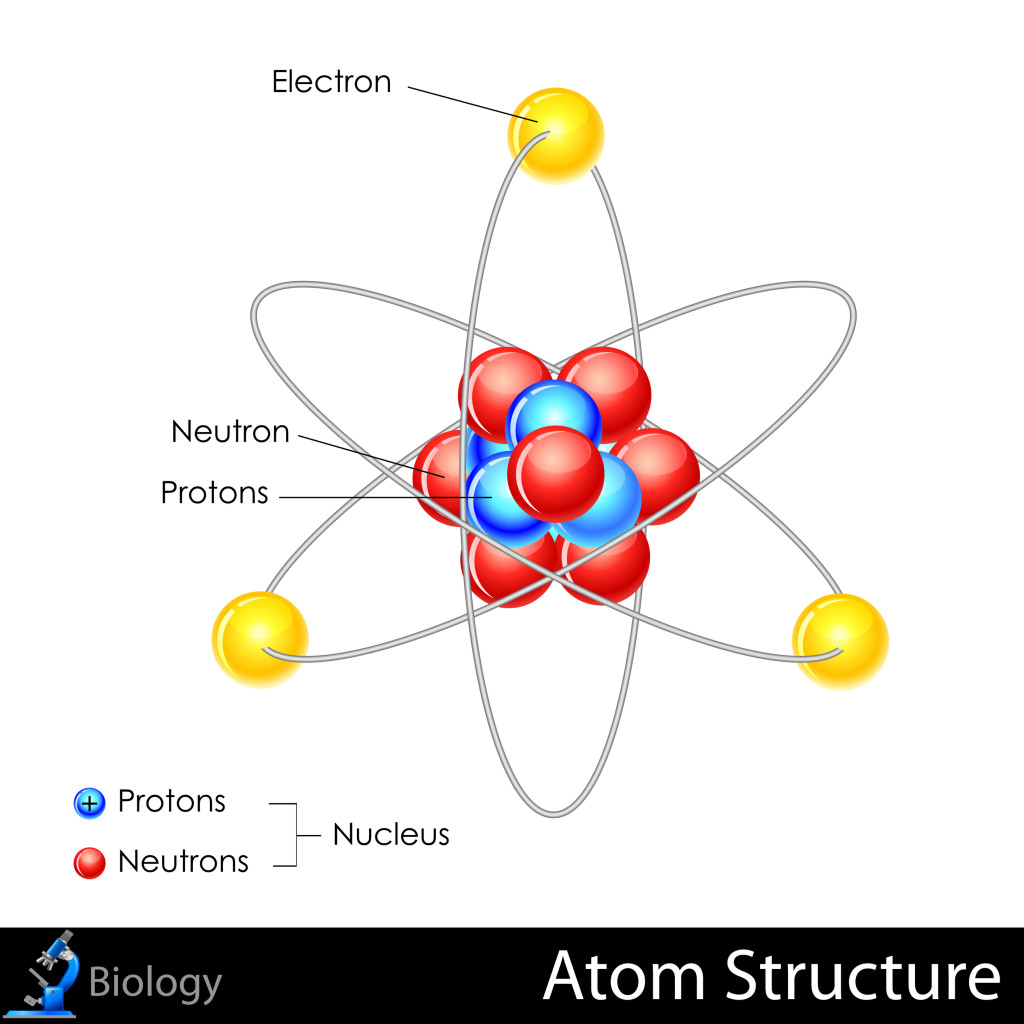
Basic Diagram of an Atom. Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. The center of an atom is the nucleus and one or more electrons surrounding the nucleus. When one says an atom is electrically neutral, it means that the number.
/GettyImages-141483984-56a133b65f9b58b7d0bcfdb1.jpg)
Basic Model of the Atom Atomic Theory
Atom Diagram [/caption]The image on the left is a basic atom diagram. This one shows the protons, neutrons, and electrons of a carbon atom. Each is in a group of six. That makes the atom very stable.
4.2 Structure of Atoms SPM Science
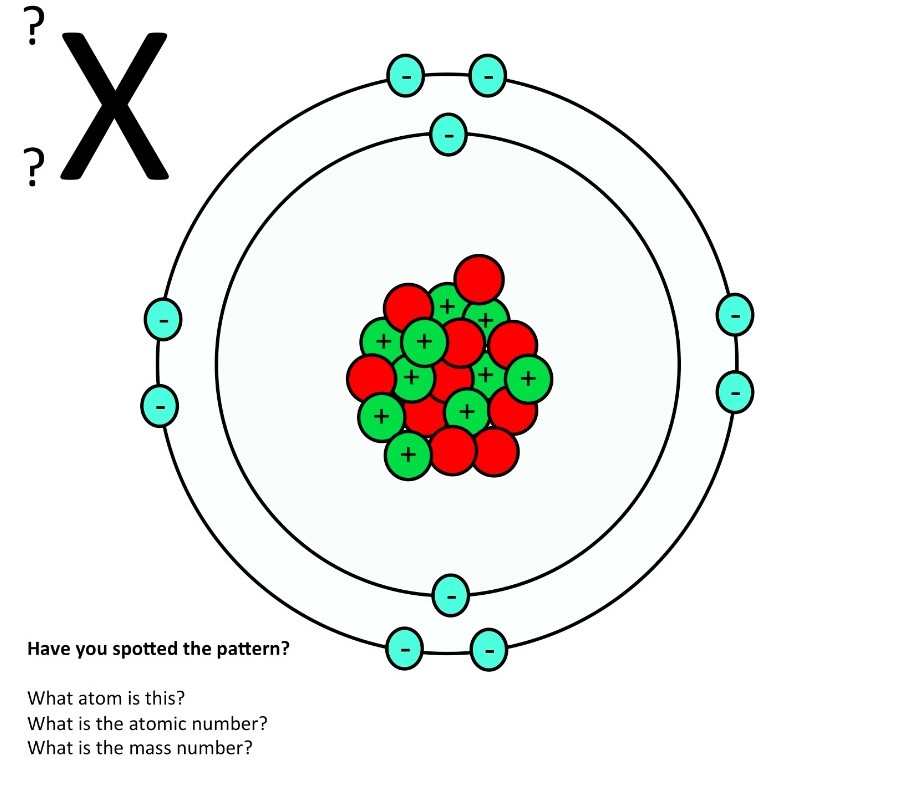
1. Draw five protons in the nucleus of the atom. Label them with their charge. 2. Draw six neutrons in the nucleus of the atom. 3. Draw two electrons in the first energy level and label them with their charge. 4. Draw three electrons in the second energy level and label them with their charge. 5. What element is represented by the diagram?

The Nucleus of the Atom and Radioactivity
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is.
The Structure of Atoms
1.14: The Nuclear Atom. Describe the history of the atom. Draw a diagram of a model of the atom and label the nucleus and the electron cloud. The history of the atom begins before 1800, where an atom was thought to be the smallest piece of matter. The first scientist to provide a theory about the atom was John Dalton.
atom diagram to label
Atom. Atoms are tiny particles that form the basic building blocks of all matter in the universe, whether solid, liquid, or gas. All living organisms and nonliving objects found on Earth are made of trillions and trillions of atoms. The smaller particles that make up an atom are known as subatomic particles. The term 'atom' was derived from.

Atomic Structure Broad Learnings
5.8: Orbitals. Page ID. Ed Vitz, John W. Moore, Justin Shorb, Xavier Prat-Resina, Tim Wendorff, & Adam Hahn. Chemical Education Digital Library (ChemEd DL) A characteristic of the diagram Figure 1 in Electron Waves in the Hydrogen Atom is that it has been assigned an identifying label, namely, 1 s.
Isotopes Definition, Explanation, Properties And Examples
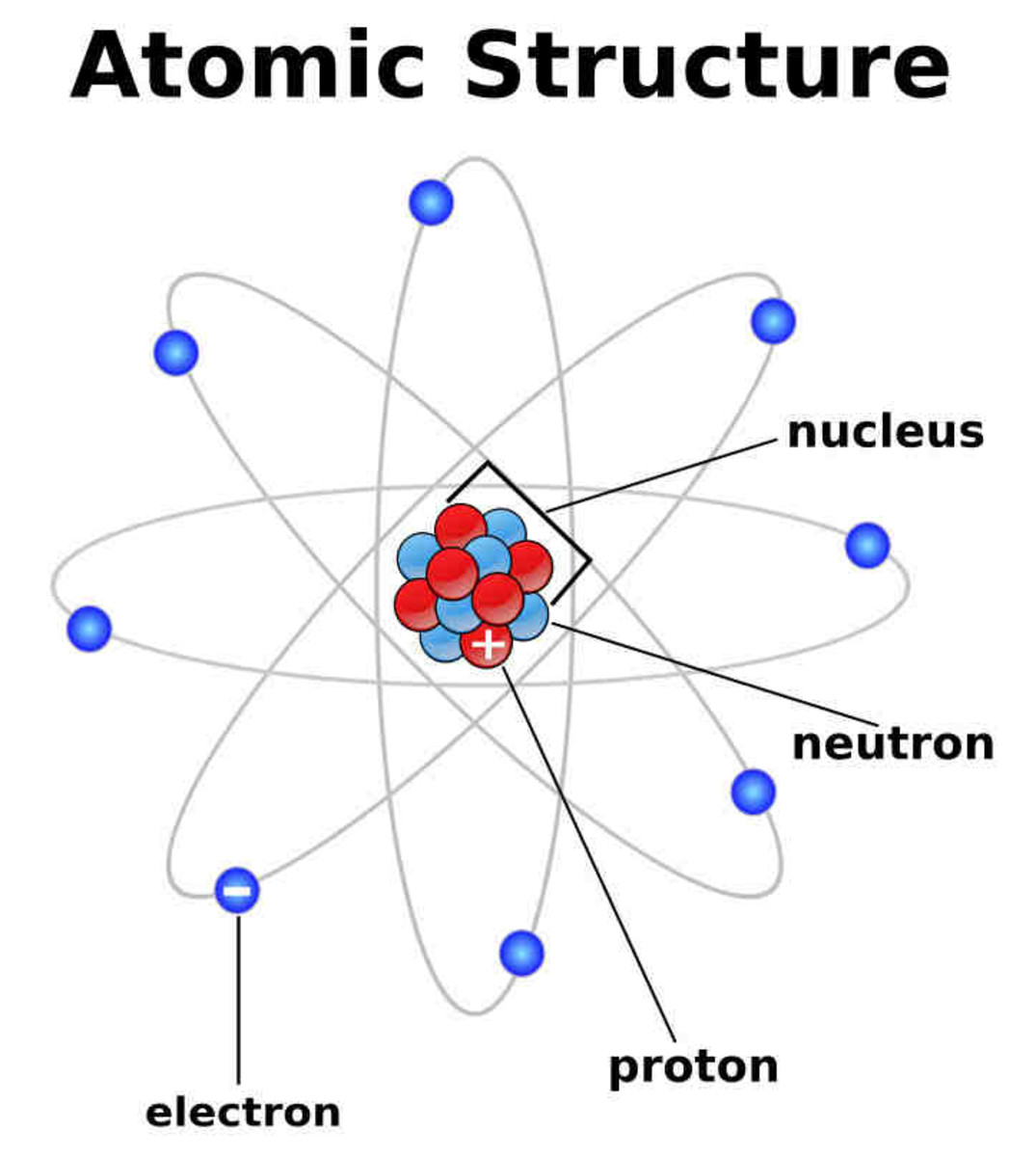
Most of the atom is empty space. The rest consists of three basic types of subatomic particles: protons, neutrons, and electrons.The protons and neutrons form the atom's central nucleus. (The ordinary hydrogen atom is an exception; it contains one proton but no neutrons.) As their names suggest, protons have a positive electrical charge, while neutrons are electrically neutral—they carry.
Atomic structure teaching resources the science teacher
The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. (It does not matter what order the positions are used.) For example, the Lewis electron dot symbol for calcium is simply. Figure 1 shows the Lewis symbols for.

Atom Definition, Structure & Parts with Labeled Diagram
An atom has a central nucleus close nucleus The central part of an atom. It contains protons and neutrons, and has most of the mass of the atom. The plural of nucleus is nuclei..This is surrounded.