
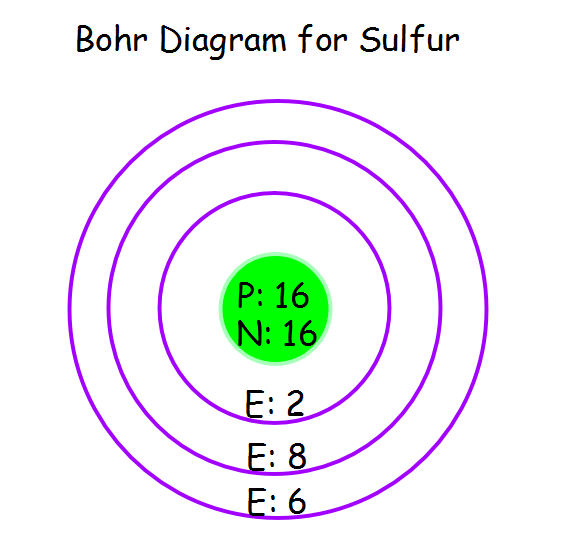
Bohr Diagram Of Sulfur
Updated on January 27, 2020 The Bohr Model has an atom consisting of a small, positively charged nucleus orbited by negatively charged electrons. Here's a closer look at the Bohr Model, which is sometimes called the Rutherford-Bohr Model. Overview of the Bohr Model Niels Bohr proposed the Bohr Model of the Atom in 1915.

Premium Vector Sulfur atom bohr model
The Bohr Model is a modification of an earlier atomic model, the Rutherford Model. The Bohr Model has an atom with a positively-charged nucleus surrounded by negatively-charged electrons that have circular, planetary-like orbits. Today, we know that the Bohr Model has some inaccuracies, but it's still used because of its simple approach to.
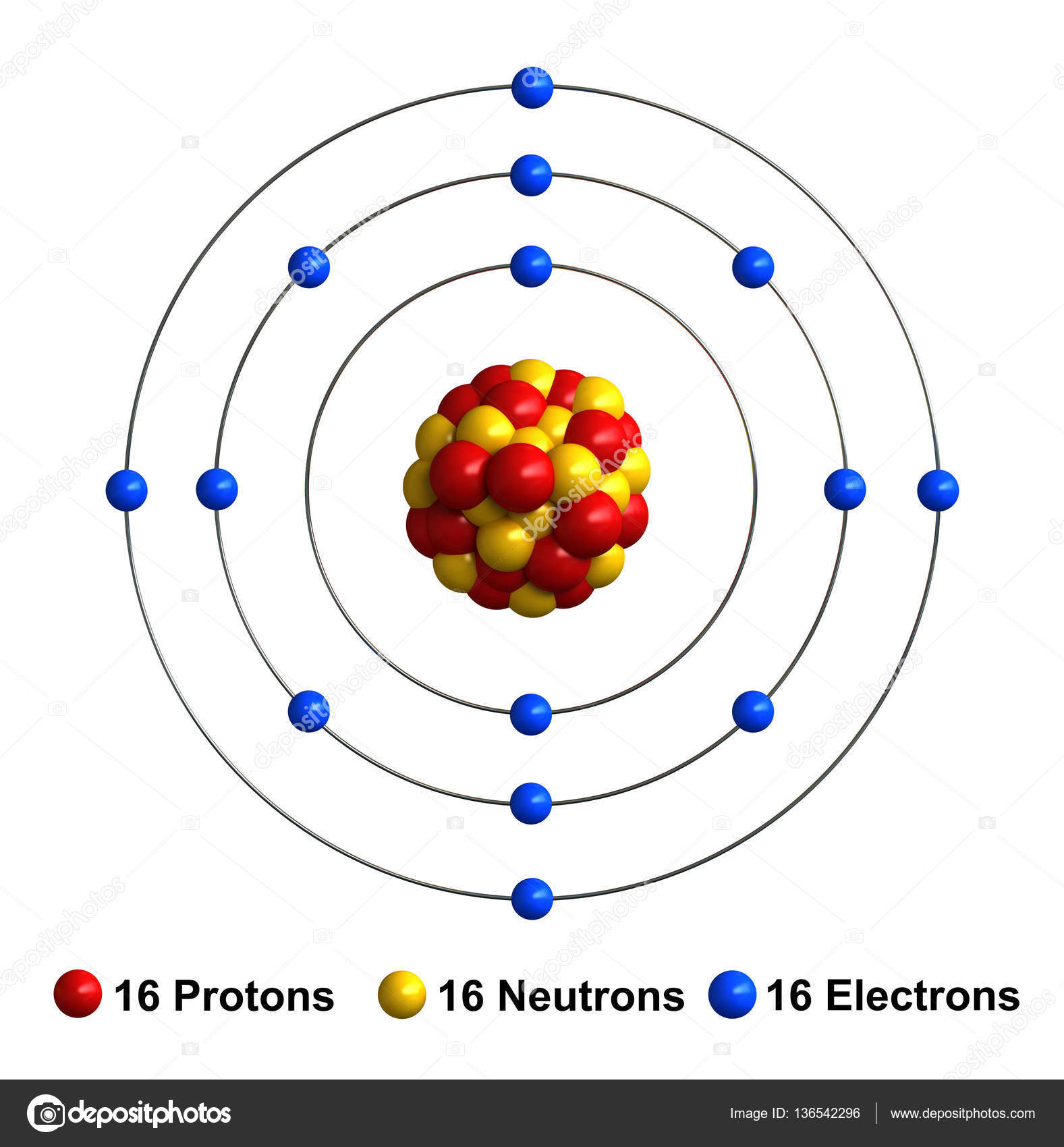
3d render of atom structure of sulfur Stock Photo by ©oorka5 136542296
In this state the radius of the orbit is also infinite. The atom has been ionized. Figure 5.4.2 5.4. 2 The Bohr Model of the Hydrogen Atom (a) The distance of the orbit from the nucleus increases with increasing n. (b) The energy of the orbit becomes increasingly less negative with increasing n.
Bohr Diagram The Element Sulfur
Describe the Bohr model of the hydrogen atom Use the Rydberg equation to calculate energies of light emitted or absorbed by hydrogen atoms

Sulfur Atom Bohr Model Vector Illustration 267662292
Learn how to draw: Sulfur Bohr model. From orbital diagram. Sulfur orbital diagram. The above orbital diagram shows that the 1s subshell has 2 electrons, the 2s subshell has 2 electrons, the 2p subshell has 6 electrons, the 3s subshell has 2 electrons, and the 3p subshell has 4 electrons.
Bohr Diagram Of Sulfur
Sulfur Bohr model The Bohr model of sulfur contains a nucleus having 16 protons and 16 neutrons in the center, and around this nucleus, there are three electron shells containing 16 electrons. Contents Steps #1 Write protons, neutrons, and electrons of sulfur atom #2 Draw nucleus of sulfur atom #3 Draw 1st electron shell #4 Draw 2nd electron shell
:max_bytes(150000):strip_icc()/sulfuratom-58b602563df78cdcd83d5a9d.jpg)
Atom Diagrams Electron Configurations of the Elements
Immediately before 1913, the Rutherford model conceived of an atom as consisting of a tiny positively charged heavy core, called a nucleus, surrounded by light, planetary negative electrons revolving in circular orbits of arbitrary radii. Britannica Quiz Matter and More Quiz How does Niels Bohr's atomic model work?
Sulfur Bohr Model Diagram My XXX Hot Girl
Physics suggests that electrons do not physically exist as "points," but teachers use the Bohr atom model with fixed electrons as a way to simplify atomic structure. Creating the model requires the ability to cut with scissors and use glue. Wear a pair of surgical gloves.

Sulfur Bohr Model — Diagram, Steps to Draw Techiescientist
The Bohr Model of Sulfur (S) has a nucleus that contains 16 neutrons and 16 protons. This nucleus is surrounded by three-electron shells named K-shell, L-shell, and M-shell. The outermost shell in the Bohr diagram of Sulfur contains 6 electrons that also called valence electrons. Page Contents show How to draw Bohr Model of Sulfur (S)?

Sulfur Bohr Model — Diagram, Steps to Draw Techiescientist
Bookshelves Physical & Theoretical Chemistry Supplemental Modules (Physical and Theoretical Chemistry) Electronic Structure of Atoms and Molecules

Sulfur atom tewswheel
The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +12 m s = + 1 2 ).
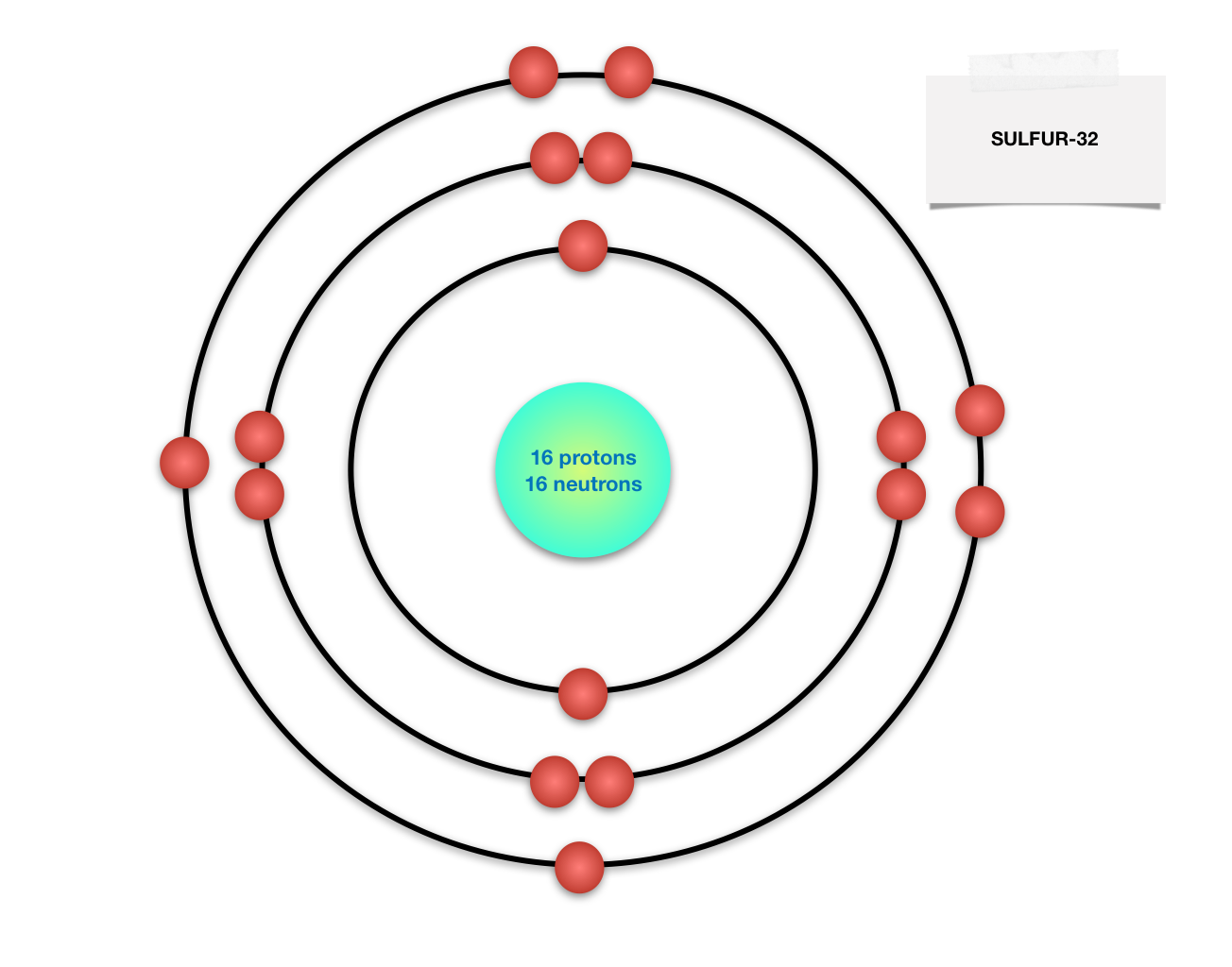
Draw BohrRutherford diagram for the sulfur32 atom. Quizlet
How to Draw the Bohr-Rutherford Diagram of Sulfur chemistNATE 247K subscribers Subscribe 415 32K views 4 years ago Sulfur / Sulphur has 2 electrons in its first shell, 8 in its second, 6 in.
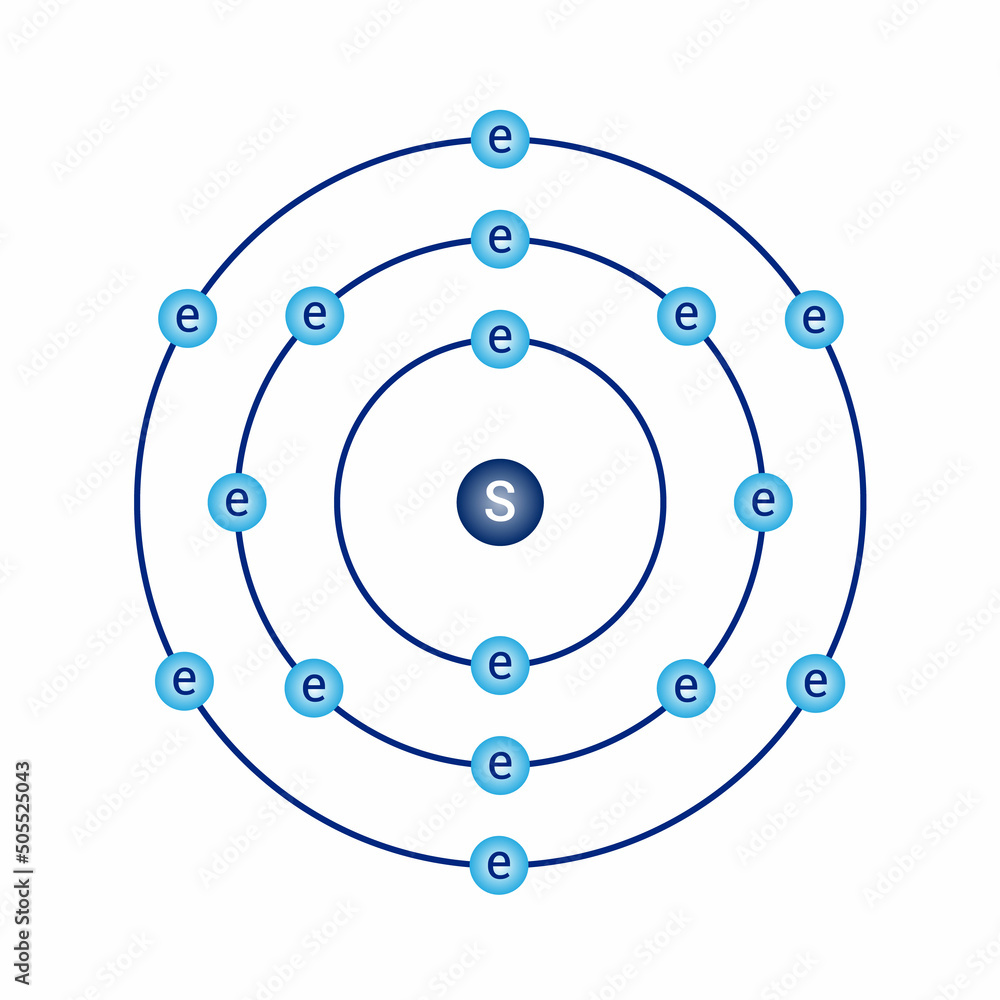
bohr model of the sulfur atom. electron structure of sulfur Stock
Applications of the Bohr Rutherford Diagram of Sulfur. The Bohr Rutherford diagram, also known as the Rutherford-Bohr model, is a simplified representation of the atomic structure of an element. It is extensively used to understand the electron configuration and predict the chemical behavior of elements. The Bohr Rutherford diagram specifically.

Diagram representation of the element sulfur Vector Image
The Bohr model of the hydrogen atom (Z = 1) or a hydrogen-like ion (Z > 1), where the negatively charged electron confined to an atomic shell encircles a small, positively charged atomic nucleus and where an electron jumps between orbits, is accompanied by an emitted or absorbed amount of electromagnetic energy (hν). The orbits in which the electron may travel are shown as grey circles; their.
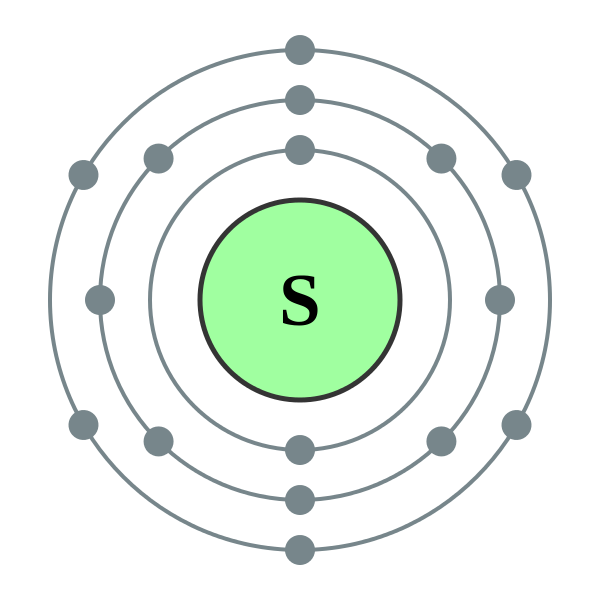
Bohr Diagram Of Sulfur
Bohr model is a structural model in which the negatively charged electrons revolve around the positively charged nucleus. This is similar to the planets revolving around the sun, except that the orbits are non-planar. The electrons move in a fixed orbits (shells) and each orbit has a fixed energy. Each orbit (or shell) can hold a certain number.

Sulfur Bohr Model — Diagram, Steps to Draw Techiescientist
In this video we'll look at the atomic structure and Bohr model for the Sulfur atom (S). We'll use a Bohr diagram to visually represent where the electrons a.