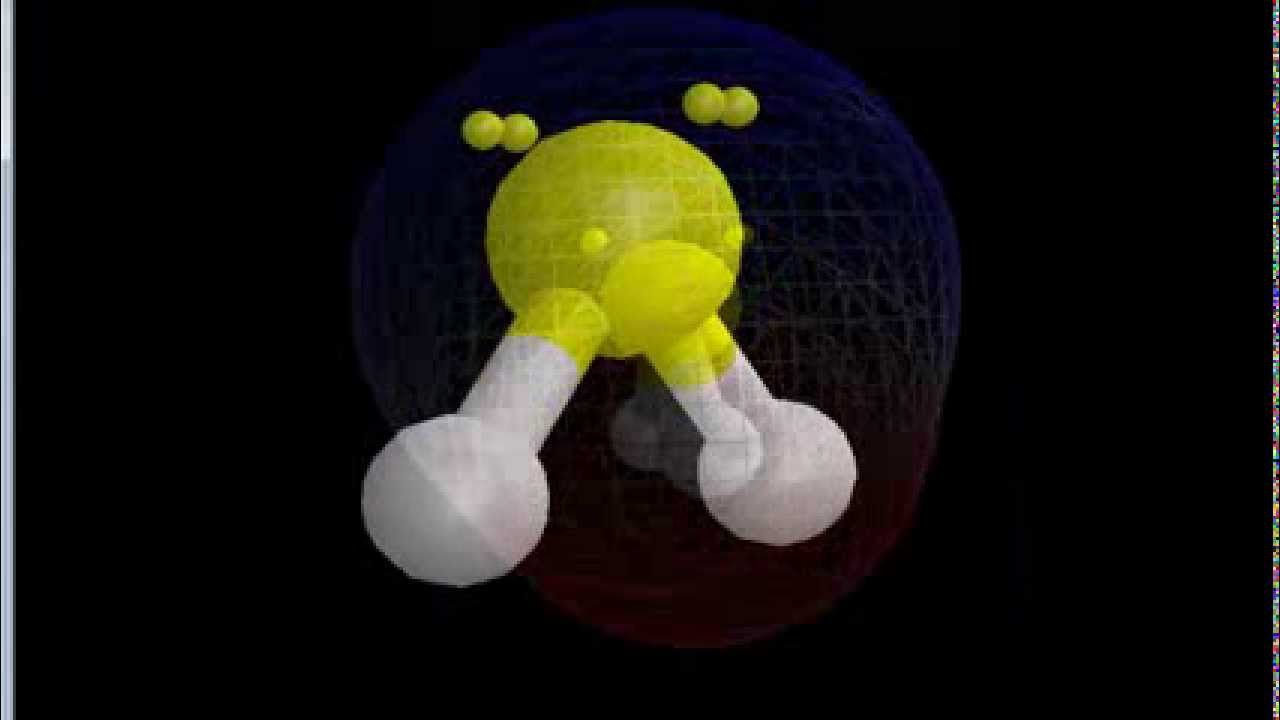
Is SF2 Polar or Nonpolar? YouTube
Which of the following molecules and ions contain polar bonds? Which of these molecules and ions have dipole moments? a. ClF 5. b. ClO−2 ClO 2 −. c. TeCl2−4 TeCl 4 2 −. d. PCl 3.
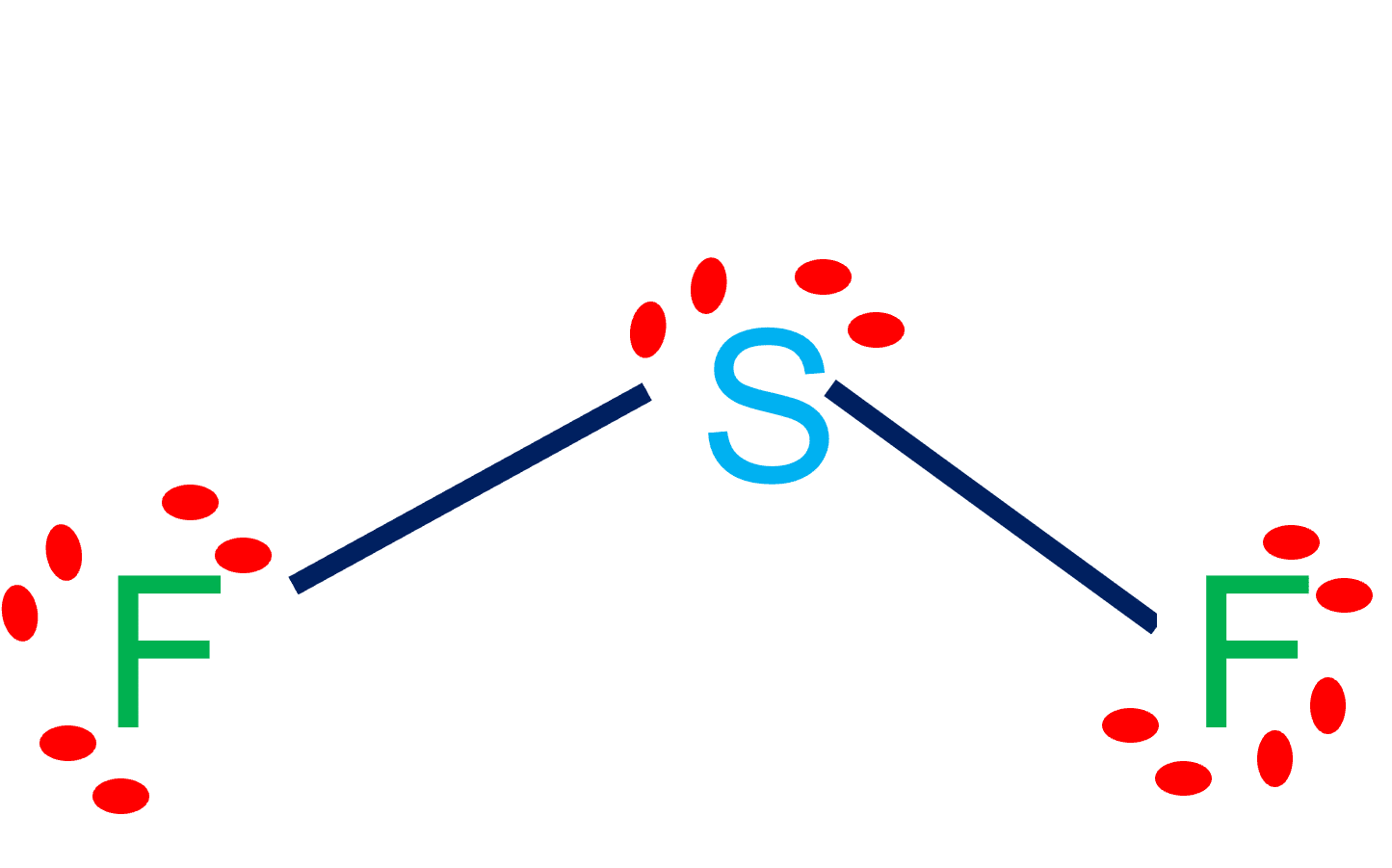
SF2 Lewis Structure & Molecular Geometry Simple Steps What's Insight
SF2 lewis structure comprises sulphur and the most electronegative element fluorine both of which belong to adjacent groups in the periodic table. Their properties are illustrated in this article. SF2 lewis structure involves 1 sulphur and 2 fluorine atoms. The sulphur atom has 6 valence electrons and the fluorine atom has 7 valence electrons.
Sf2 polar or nonpolar jujapress
Which of these molecules and ions contain polar bonds? Which of these molecules and ions have dipole moments?SF2OpenStax™ is a registered trademark, which wa.
Solved Which of the following molecule is polar? O SF2 O CO2
SF2 is polar in nature because the sulfur (2.58) and fluorine (3.98) atoms in the molecule differ in their electronegativity and the molecule has a bent geometrical shape. Therefore, the dipoles of the S-F bond do not cancel out each other and molecules turn out to be polar and contribute some dipole moment.

SF2 Lewis structure, Molecular geometry, Hybridization, Polar or nonpolar
Question: One of these molecules is SF2, and one is BeF2. Is A correctly labeled BeF2? Is SF2 polar? Is BeF2 polar? Short Answer Toolbar navigation BIUS E A A ! This question will be sent to your instructor for grading « Prev 10 of 25 Next >

So far, we’ve used 20 of the SF2 Lewis structure’s total 20 outermost
Is SF2 polar or nonpolar? SF2 Valence electrons For drawing the Lewis structure for any molecule, we first need to know the total number of valence electrons. So we will first find out the total valence electrons for Sulphur Difluoride. Total number of valence electrons for SF2 - Valence electrons of Sulphur + Valence electrons of Fluorine

Three monosulfur fluorides are observed SF2, SF4, and SF6. Of these
It is an inorganic compound with the chemical formula SF2. In this article, we will discuss Sulfur difluoride (SF2) lewis structure, molecular geometry, bond angle, polar or nonpolar, its hybridization, etc. Sulfur difluoride is formed by the reaction between one mole of sulfur dichloride with 2 moles of potassium fluoride.

Solved Which of the following molecule is polar? O SF2 O CO2
Answer. 4.10: Polar Molecules is shared under a CC BY-NC-SA 3.0 license and was authored, remixed, and/or curated by LibreTexts. The molecular polarity of a diatomic molecule is determined by the bond polarity. The polarity of molecules with more than one bond must be determined by first identifying the molecular structure and..

Sf2 polar or nonpolar limfamike
1.44. Carbon Dioxide. 0. H. 2.20. Figure 8.8.1 8.8. 1: Both carbon dioxide and water have polar bonds, but only water is a polar molecule. Carbon dioxide is symmetric and the pull of the two oxygens on the carbon's electrons cancel out, so it is a nonpolar molecule with polar bonds.

Is SF2 Polar or Nonpolar? Techiescientist
Is SF2 Polar or Nonpolar? Wayne Breslyn 665K subscribers Subscribe 33K views 9 years ago If you look at the Lewis structure for SF2 might appear to be a symmetrical molecule. However, according.
[Solved] SF2 does this compound contain polar covalent bond or nonpolar
Is S F 2 polar or nonpolar? Explain. Polar Molecule: There are many covalent chemical compounds that are known to be polar. This means that their molecular unit contains a net dipole moment,.

Is SF2 Polar or Nonpolar? (Sulfur Difluoride) Polar, Chemical formula
Sulfur Fluoride is a highly unstable inorganic compound. With a molar mass of 70.062 g/mol, this compound is made up of one Sulfur atom and two Fluoride atoms. This compound is formed when sulfur dichloride reacts at low pressure with either potassium fluoride or mercury (||) fluoride. SCl2 + 2KF —-> SF2 + 2 KCl SCl2 + HgF2 ——-> SF2 + HgCl2
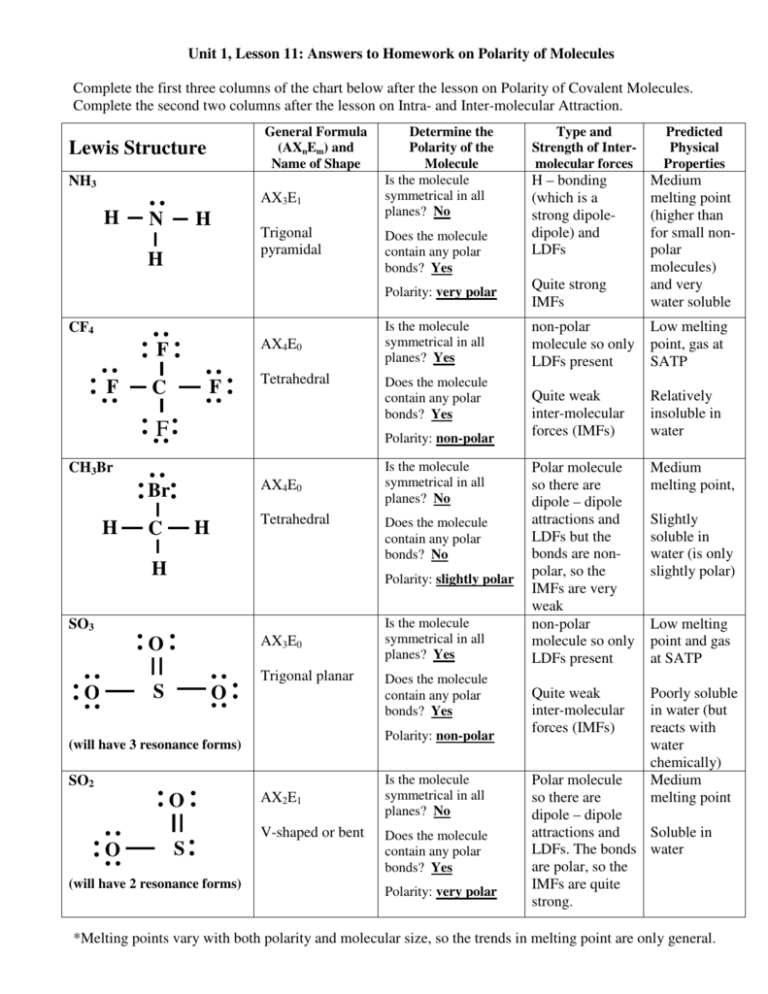
Sf2 polar or nonpolar lenaka
SF2 lewis structure comprises sulphur and the most electronegative element fluorine both of which belong to adjacent groups in the periodic table. Their properties are illustrated in this article. SF2 lewis structure involves 1 sulphur and 2 fluorine atoms. The sulphur atom has 6 valence electrons and the fluorine atom has 7 valence electrons.

Sf2 molecule polar or nonpolar? [Expert Review]
Sulfur difluoride (SF2) is a highly unstable inorganic compound. SF2 lewis structure comprises one Sulfur Atom and two Fluoride atoms. It is a polar molecule with bond angles of 98 degrees. Table of Contents Step by Step Construction of Lewis Structure SF2 Molecular Geometry SF2 Lewis Structure- Key Points SF2 Hybridization
SOLVED Kepolaran suatu senyawa kovalen bergantung pada. * 4 poin a
SF2) molecule is classified as a polar molecule. The molecule of sulfur difluoride (with tetrahedral or bent V-shaped molecular geometry) is tilted, the bond angles between sulfur and . fluorine are 98.3 degrees. It has a difference in electronegativity values between sulfur and .

Is SF2 Polar or Nonpolar? Techiescientist
SF2 is a POLAR molecule. But why? And how can you say that SF2 is a polar molecule? Want to know the reason? Let's dive into it! SF2 is a POLAR molecule because the Fluorine (F) present in the molecule is more electronegative, which causes the partial positive (ẟ+) and partial negative (ẟ-) charge to appear on the molecule.