Sbr2 Dot Structure
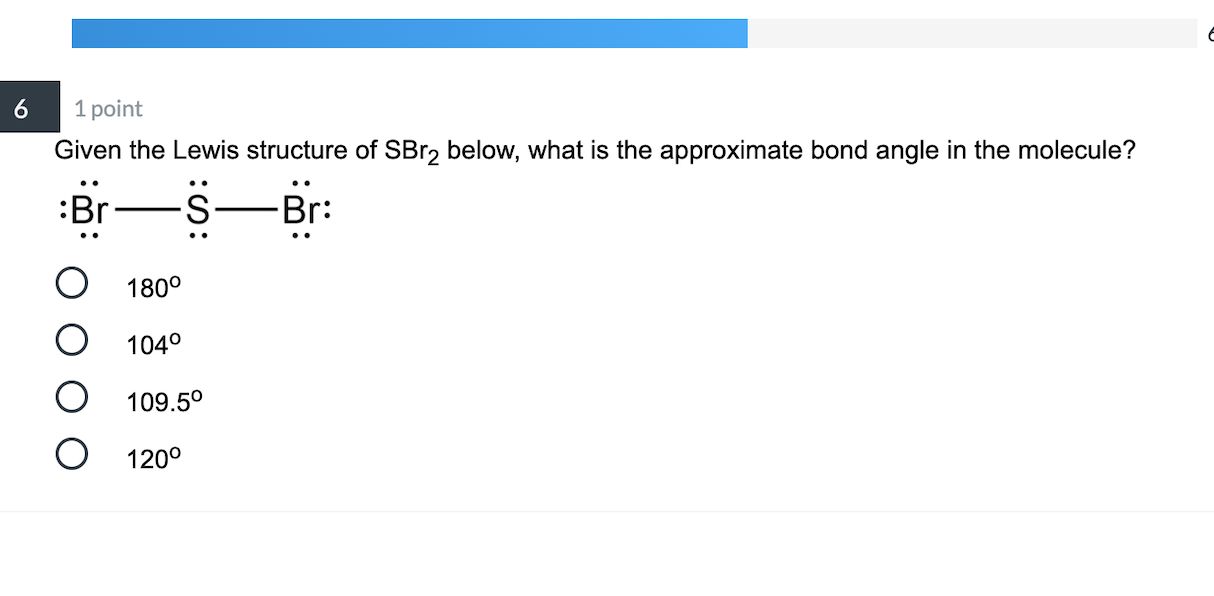
Question: Draw the Lewis structure of SBr2 and use it to answer the following questions. What is the electron domain geometry of this molecule? (Select] What is the molecular geometry of this molecule? [Select) Is this molecule polar? [Select) What is the approximate value of the Br-S-Br bond angle in this molecule? (Select) What is the bond.
Sbr2 Dot Structure
Textbook Question. Values of Ea = 6.3 kJ>mol and A = 6.0 * 108>1M # s2 have been measured for the bimolecular reaction: NO1g2 + F21g2S NOF1g2 + F1g2 (b) The product of the reaction is nitrosyl fluoride. Its formula is usually written as NOF, but its structure is actually ONF.

Lewis Dot Structure For Sbr2
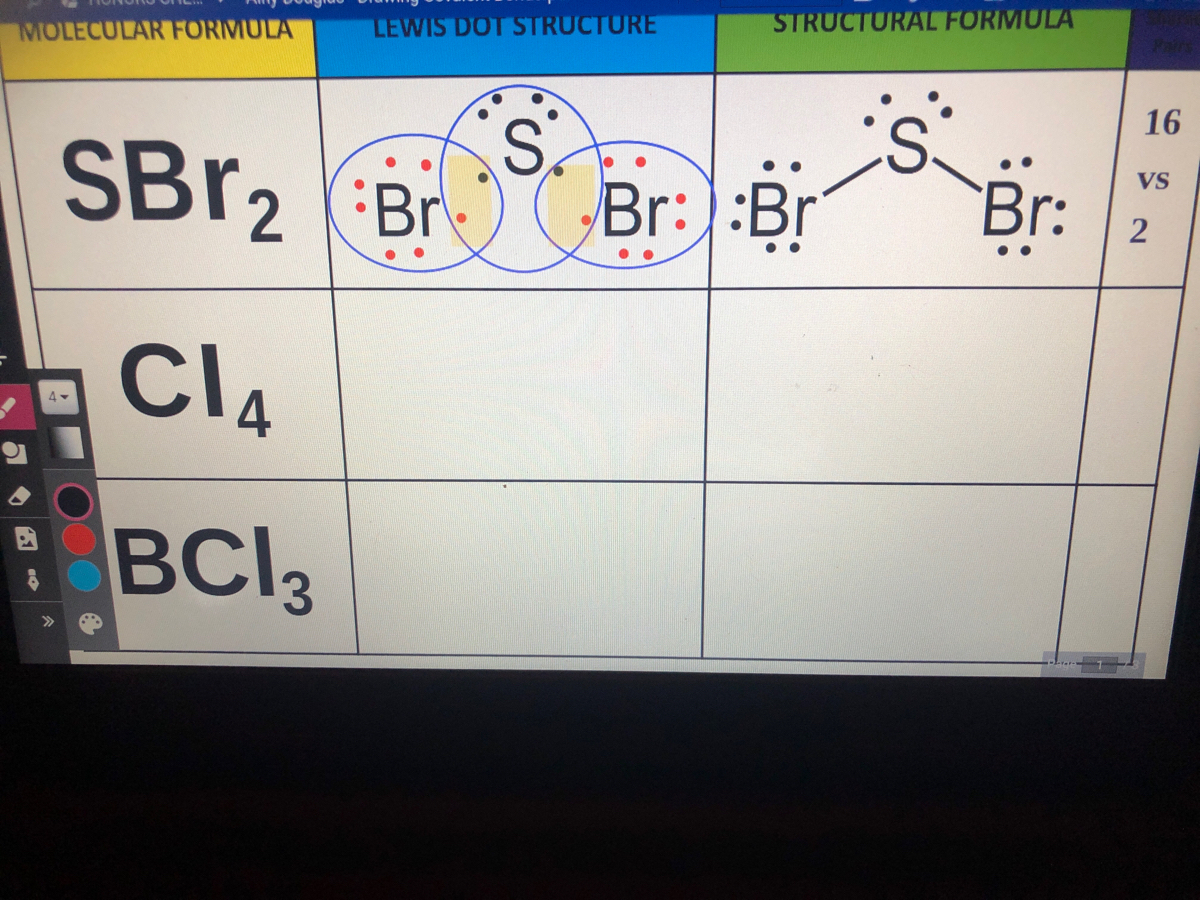
Lewis structure of SBr2 contains two single bonds between the Sulfur (S) atom and each Bromine (Br) atom. The Sulfur atom (S) is at the center and it is surrounded by 2 Bromine atoms (Br). The Sulfur atom has 2 lone pairs and both the Bromine atoms have 3 lone pairs. Let's draw and understand this lewis dot structure step by step.
Ajeet Sylus And Alex Garcia's Lewis Dot Structure Science ShowMe
To understand its molecular geometry, we first look at its Lewis Structure and shape..

Sbr2 Lewis Dot Structure
Sulfur dibromide (SBr2) has the composition of one sulfur and two bromine atoms. What is the molecular geometry of sulfur dibromide?. Drawing and predicting the SBr2 molecular geometry is very easy by following the given method. Here in this post, we described step by step to construct SBr2 molecular geometry.
Sbr2 Dot Structure
SBr2 Lewis structure. November 7, 2023 by Deep. The information on this page is fact-checked. SBr 2 Lewis structure. SBr 2 (sulfur dibromide) has one sulfur atom and two bromine atoms. In the SBr 2 Lewis structure, there are two single bonds around the sulfur atom, with two bromine atoms attached to it. Each bromine atom has three lone pairs.

Download Sf2 Lewis Structure Molecular Geometry Images GrAffiTi
The Lewis structure for SBr 2 is fairly straighforward and only involves single bonds. For the SBr 2 Lewis structure there are a total of 20 valence electrons available. SBr 2 is similar to the SCl 2 Lewis structure. SBr2 Lewis Structure - How to Draw the Dot Structure for SBr2 (Sulfur dibromide) Watch on See the Big List of Lewis Structures
Sbr2 Dot Structure
Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. For math, science, nutrition, history.
Electron Geometry Of Sbr2? Yahoo Answers Online encyclopedia
The first step is to sketch the Lewis structure of the SBr2 molecule, to add valence electrons around the sulfur atom; the second step is to add valence electrons to the two bromine atoms, and the final step is to combine the step1 and step2 to get the SBr2 Lewis Structure.
Solved 6 1 point Given the Lewis structure of SBr2 below,
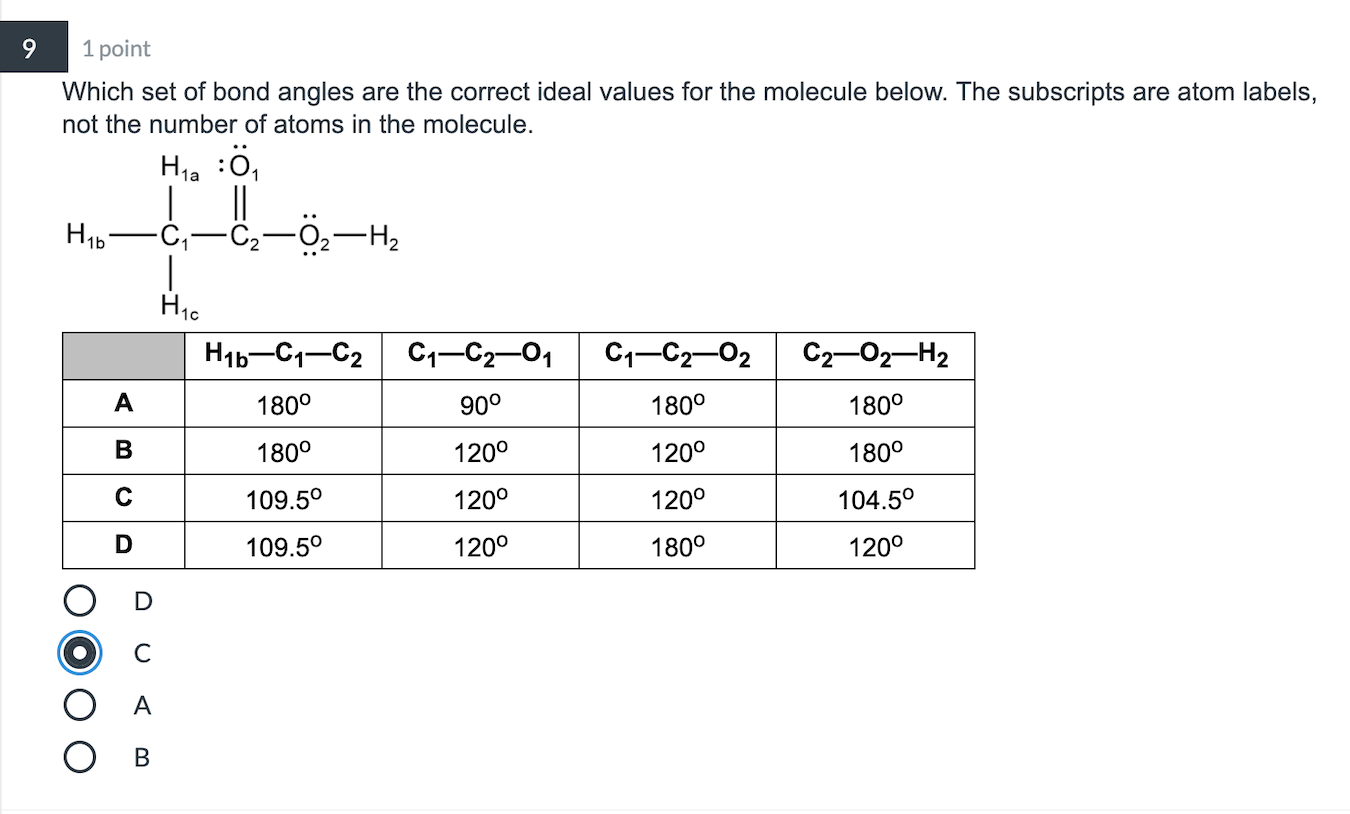
1. The central atom, sulfur, contributes six valence electrons, and each fluorine atom has seven valence electrons, so the Lewis electron structure is. With an expanded valence, that this species is an exception to the octet rule. 2. There are six electron groups around the central atom, each a bonding pair.

Calculating SBr2 Formal Charges Calculating Formal Charges for SBr2
A step-by-step explanation of how to draw the SBr2 Lewis Dot Structure (Sulfur dibromide).For the SBr2 structure use the periodic table to find the total num.

Solved Molecule Sulfur dibromide, SBr2 Lewis Structure
The SBr2 Lewis structure refers to the arrangement of atoms and electrons in a molecule of sulfur dibromide. In this structure, there are two bromine atoms bonded to a central sulfur atom. The Lewis structure helps us understand the bonding and electron distribution within the molecule.
SBr2 electron dot diagram Science, Chemistry, Elements, Periodic
What is the Lewis structure of [//substance:SBr2//]? Natural Language Math Input Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. For math, science, nutrition, history, geography, engineering, mathematics, linguistics, sports, finance, music…

So far, we’ve used 20 of the SBr2 Lewis structure’s total 20 outermost
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

Sbr2 Lewis Dot Structure
SBr2 lewis structure has a Sulfur atom (S) at the center which is surrounded by two Bromine atoms (Br). There are 2 single bonds between the Sulfur atom (S) and each Bromine atom (Br). There are 2 lone pairs on the Sulfur atom (S) and 3 lone pairs on both the Bromine atoms (Br).
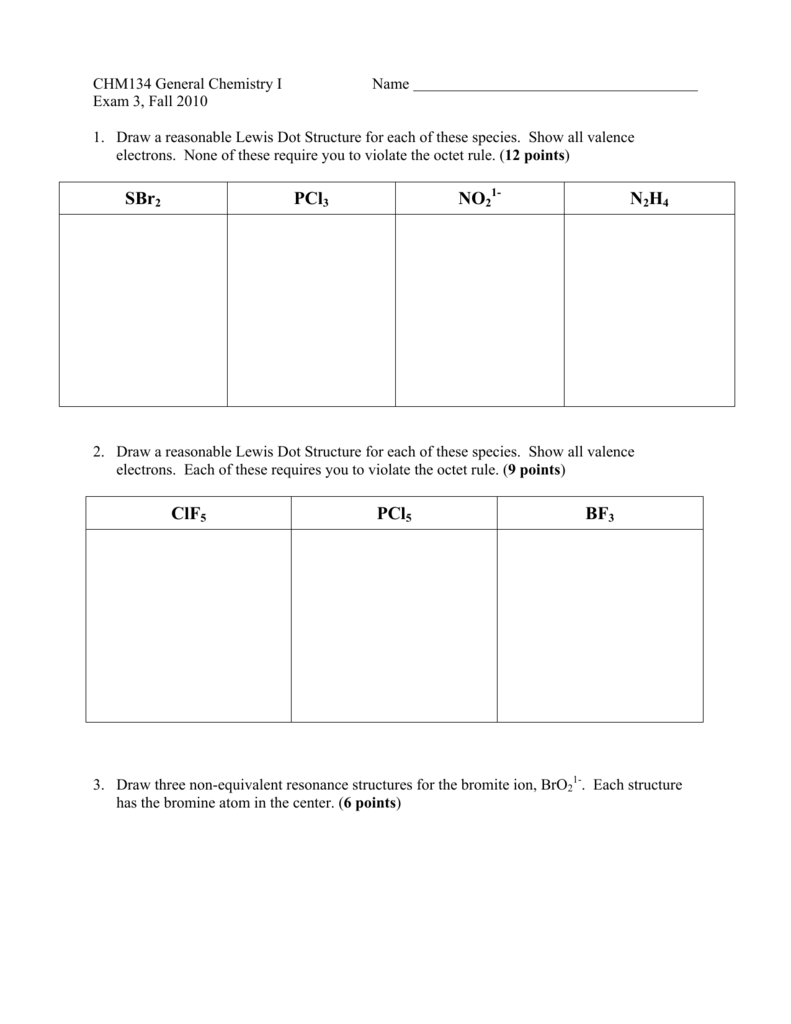
SBr2 PCl3 NO2 N2H4 ClF5 PCl5 BF3
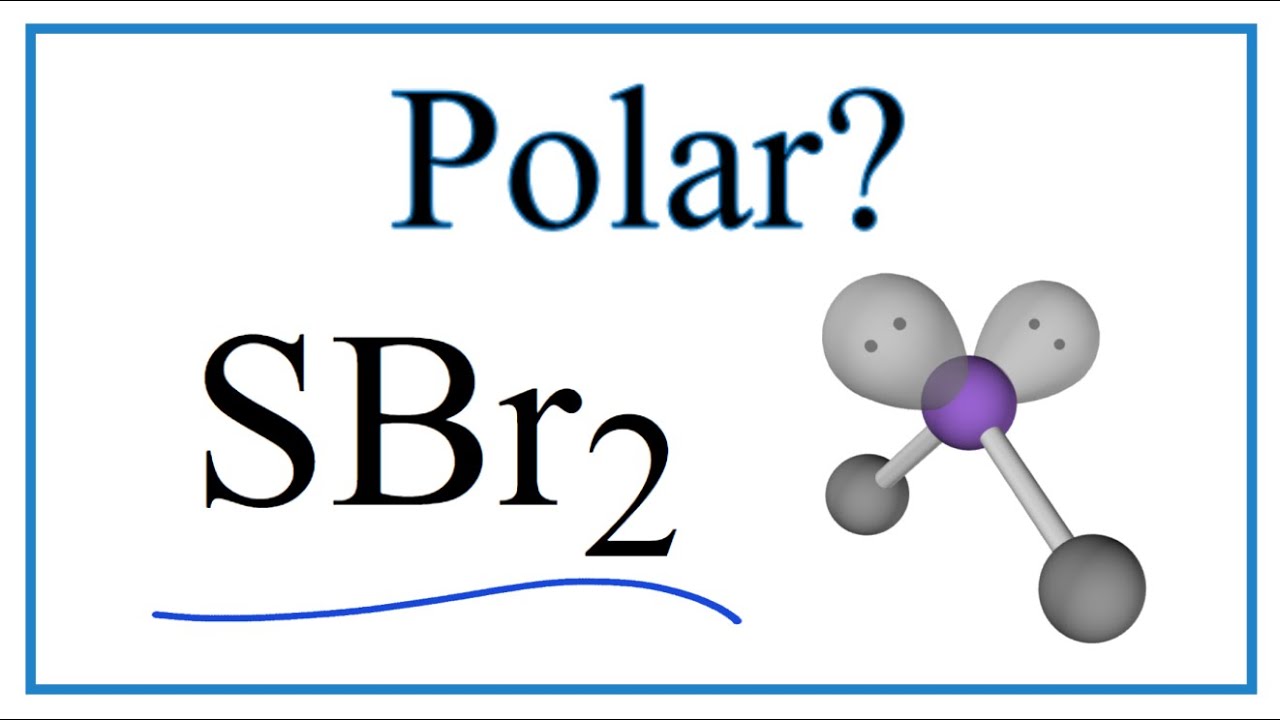
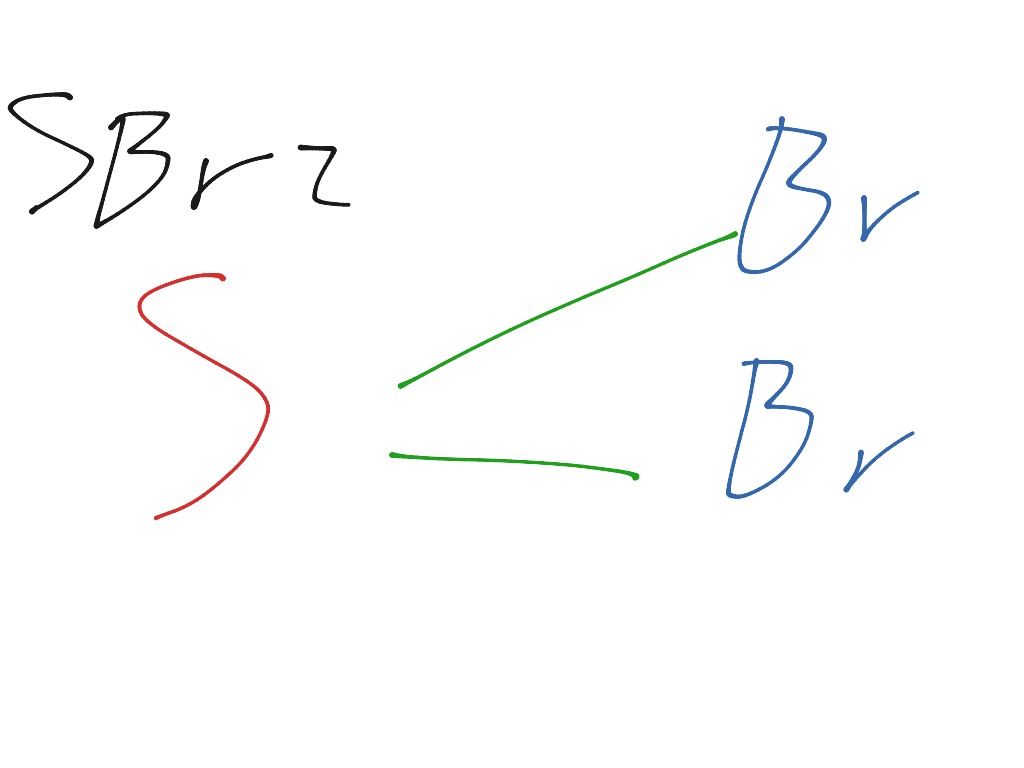
A quick explanation of the molecular geometry of SBr2 including a description of the SBr2 bond angles.Looking at the SBr2 Lewis structure we can see that the.