
What is the lowest temperature setting on Masterbuilt electric smoker
Boiling points of common materials. Boiling point of water: 100 °C / 212 °F Boiling point of water (in Kelvin): 373.2 K Boiling point of ethanol: 78.37 °C / 173.1 °F Boiling point of methanol: 64.7 °C / 148.5 °F Boiling point of acetone: 56 °C / 132.8 °F Boiling point of alcohol: 78.37 °C / 173.1 °F Boiling point of nitrogen: -195.8.

An acidcatalyzed irreversible liquidphase reaction A B is carried out
This means that the temperature at which a liquid becomes a gas, the boiling point, can change with surrounding pressure. Therefore, we define the normal boiling point as the temperature at which a liquid changes to a gas when the surrounding pressure is exactly 1 atm, or 760 torr. Unless otherwise specified, it is assumed that a boiling point.

ProShares Ultra Bloomberg Natural Gas (BOIL) Stock Surges on Increasing
10.12: Boiling Point. When we heat a liquid until it boils, the bubbles that form inside the liquid consist of pure vapor. If the liquid is well stirred while boiling occurs, the vapor in the bubbles will be in equilibrium with the liquid and will have a pressure equal to the vapor pressure at the boiling temperature.
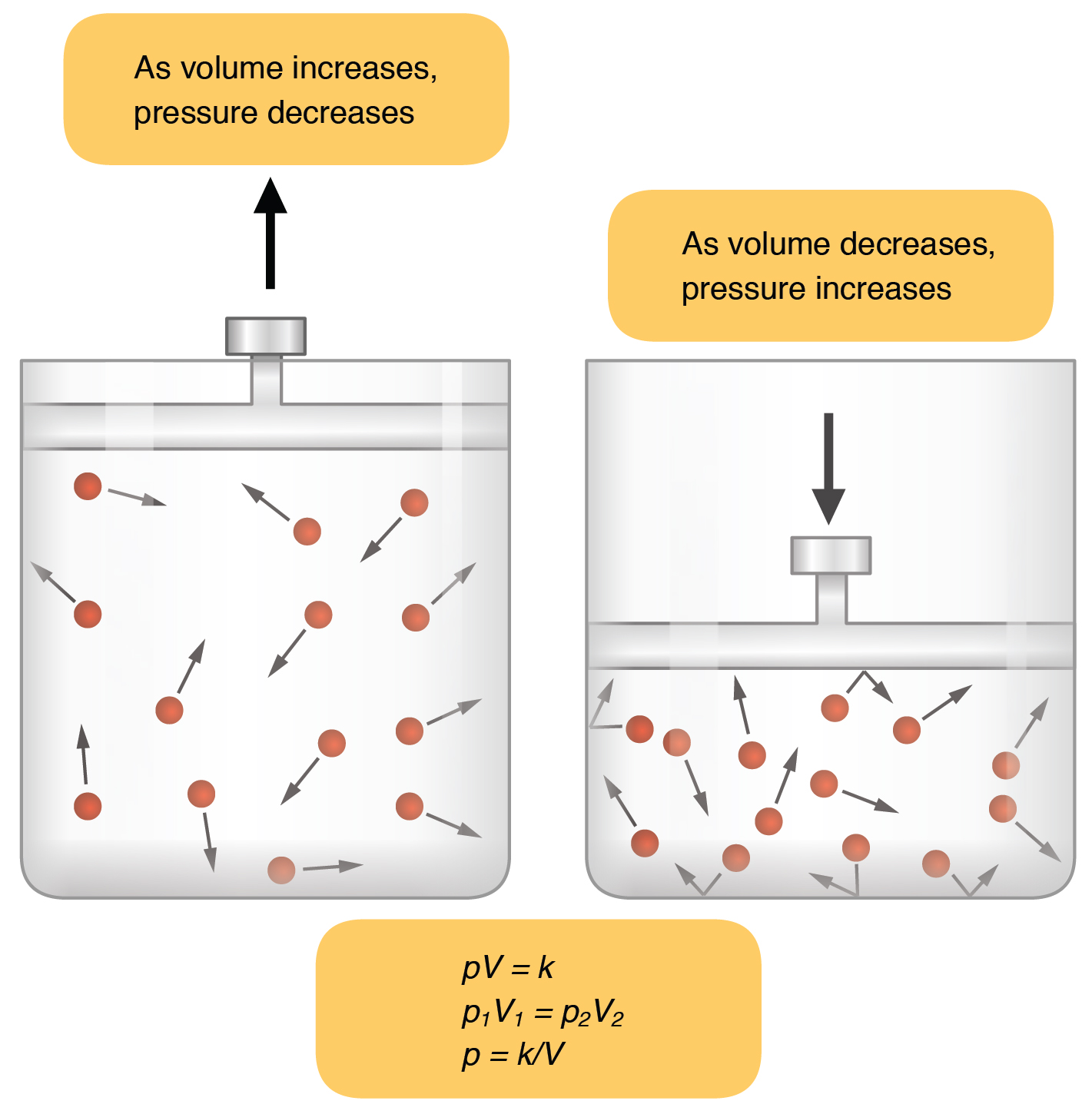
A sample of a gas has a volume of 2.0 liters at a pressure of 1.0
The temperature of liquid nitrogen is −195.79 °C (77 K; −320 °F). Liquid nitrogen has many uses, but poses risks of frostbite, explosion, and suffocation if handled incorrectly. Liquid nitrogen is very cold! At room temperature and pressure, liquid nitrogen boils into nitrogen gas. It even looks like boiling water, except that it is.
What temperature does overheat protection kick in? Any way to check its
Ethanol boils at 178.5 degrees F, while water boils at 212 degrees F. The trick in a carburetor is to make the fuel evaporate into fumes when atomized without lowering the boiling point.

Boiling Point of Water What Temperature Does Water Boil?
The temperature at which a liquid boils and turns into a gas is called the boiling point. The boiling point temperature will be lower if the atmospheric pressure is decreased. For example, the boiling point of pure water at standard atmospheric pressure (or sea level) is 100°C (212°F), while at 10,000 feet (3,048m), it is 90.39° C (194.7°F).

What Temperature Does Water Boil? Your Culinary Compass
Joined 17 years ago. 14,019 Posts. With the assistance of Google, I found that the boiling point of ethanol is 173.1F - but the boiling point of gasoline isn't as clear, with that boiling point being between 100-400F depending on additives, grade of fuel, etc.

ETi 400 High Efficiency Gas Pool Heater TradeGrade
Specifications define the temperatures at which. various percentages of the fuel are evaporated. Distillation limits include maximum temperatures that 10% is evaporated (50-70C), 50% is. evaporated (110-121C), 90% is evaporated (185-190C), and the final boiling point (225C). A minimum temperature for 50% evaporated (77C), and a maximum.

Real Gas vs Ideal Gas
The standard boiling point has been defined by IUPAC since 1982 as the temperature at which boiling occurs under a pressure of one bar. [6] The heat of vaporization is the energy required to transform a given quantity (a mol, kg, pound, etc.) of a substance from a liquid into a gas at a given pressure (often atmospheric pressure).

How Much does Water Cool when Pouring?
At standard atmospheric pressure, the propane boiling point is -44°F (-42°C). It will liquify when the temperature drops below -44°F and revert to its gas form if the temp reaches -44°F or higher. For instance, at -45 degrees F, this fuel will remain in a liquid state. One interesting fact: in most appliances, the propane can stay liquid.

Temperature sensing ingridscience.ca
The boiling point of a liquid varies according to the applied pressure; the normal boiling point is the temperature at which the vapour pressure is equal to the standard sea-level atmospheric pressure (760 mm [29.92 inches] of mercury). At sea level, water boils at 100° C (212° F). At higher altitudes the temperature of the boiling point is.
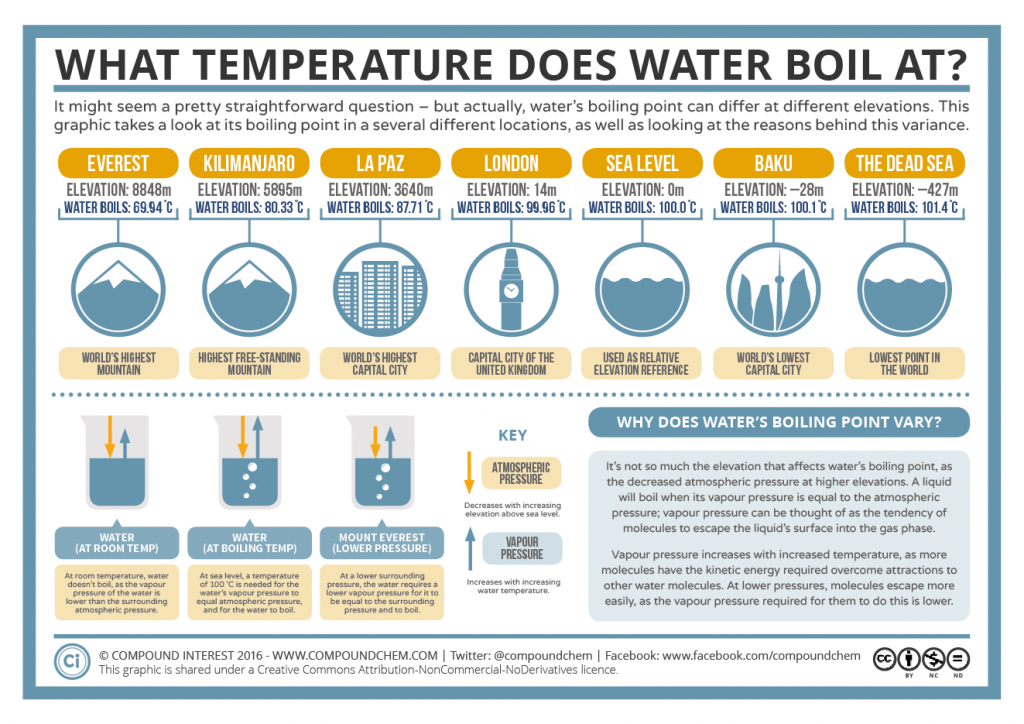
What Temperature Does Water Boil At? Boiling Point & Elevation
At the boiling point molecules anywhere in the liquid may be vaporized. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. The boiling point at atmospheric pressure (14.7 psia, 1 bar absolute) for some common fluids and gases can be found from the.
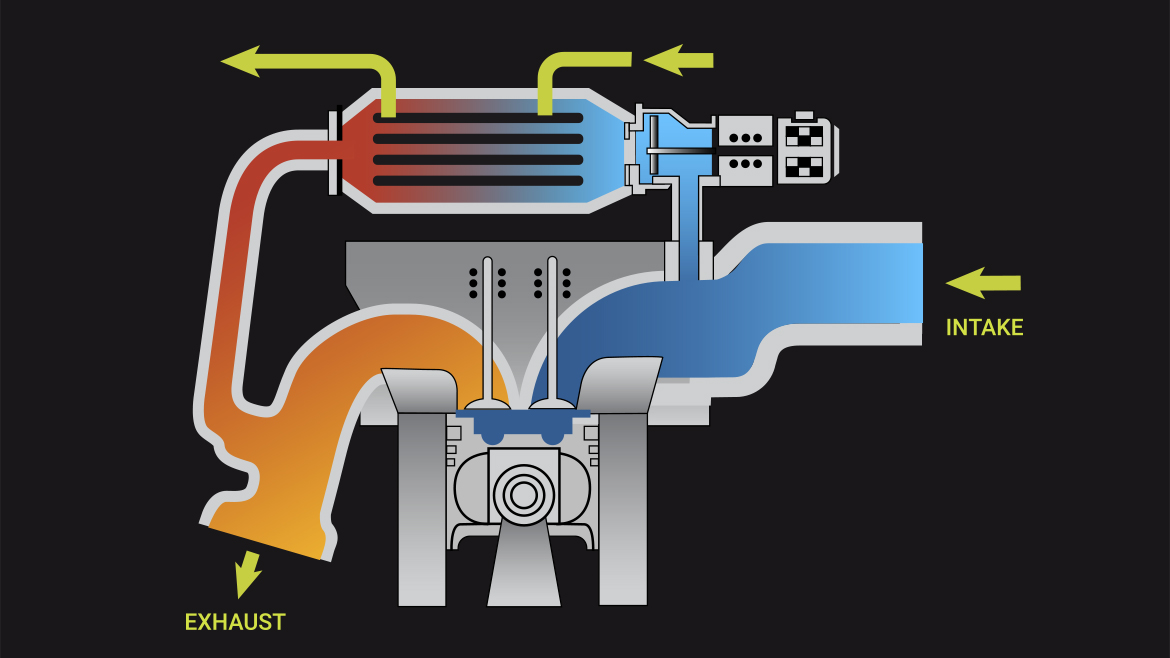
How it Works Exhaust Gas Recirculation
The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. The figure below illustrates the boiling of liquid. Figure 13.9.2 13.9. 2: Comparison between evaporation and boiling. (Credit: Christopher Auyeung; Source: CK-12 Foundation; License: CC BY-NC 3.0 (opens in new window))

At what temperature does gas freeze? YouTube
Engineering. David. Etukudo. Share. At atmospheric pressure, gasoline has an initial boiling point of 95 °F (35 °C) and a final boiling point of 395 °F (200 °C). This wide range is due to its variety of blends which alter its boiling point value. Also, pressure is another factor that alters gasoline's boiling point.
What temperature does alcohol boil? Quora
Therefore the temperature of the liquid remains constant during boiling. For example, water will remain at 100ºC (at a pressure of 1 atm or 101.3 kPa) while boiling. A graph of temperature vs. time for water changing from a liquid to a gas, called a heating curve, shows a constant temperature as long as water is boiling.

At what temperature does water boil? Trivia Answers
The boiling point of a substance is the temperature at which it can change state from a liquid to a gas throughout the bulk of the liquid. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. Some fuels and their boiling points at atmospheric pressure.